Introduction
Colorectal cancer is one of the most common cancers worldwide [1]. Since laparoscopic surgery was first applied in colorectal cancer in 1991, the technique has spread worldwide [2]. Compared to open surgery, laparoscopic surgery for rectal cancer is safe and feasible with comparable short-term outcomes and long-term outcomes [3–6].
Since the principles of total mesorectal excision (TME) were first described by Heald et al. in 1982 [7], it has become a standard procedure for rectal cancer, and reduced the local recurrence to less than 5% [8–10]. However, there remained some difficulties in middle or low rectal cancer, especially in a low location, obese patients, or males with a deep, narrow pelvis. In 2010, the down-to-up approach, transanal total mesorectal excision (TaTME), was introduced to solve these problems [11–13]. And then, there were several randomized controlled trials focusing on middle and low rectal cancer compared TaTME with laparoscopic TME (LaTME) [14, 15].
Previous meta-analyses had demonstrated a relative merit of TaTME over LaTME [16–20]. However, these studies had a relatively small sample size. What is more, some previous meta-analyses included data from abdominoperineal resections, which may generate bias, affecting outcomes [18]. Hence, we conducted this meta-analysis to assess and compare the short-term outcomes of TaTME with LaTME for middle and low rectal cancer. Intraoperative outcomes, postoperative outcomes, oncological outcomes and local recurrence were measured with meta-analytical methods.
Aim
The aim of the study was to assess and compare the short-term outcomes of TaTME with conventional LaTME for middle and low rectal cancer.
Material and methods
This meta-analysis adheres to the Preferred Reporting Items for Systematic Reviews and Meta-analysis and Meta-analysis guidelines [21, 22].
Literature-search strategy
Literature searches of PubMed, Embase and Cochrane Library databases for studies addressing TaTME versus conventional LaTME for rectal cancer between 2008 and December 2018 were performed. Only English-language publications were involved. The search terms were “Transanal or transanal total mesorectal excision or TaTME or transanal minimally invasive surgery or TAMIS or transanal endoscopic microsurgery or TEM or natural orifice transluminal endoscopic surgery or NOTES or natural orifice specimen extraction or NOSE or transanal specimen extraction” and “rectal cancer or proctectomy”.
Inclusion and exclusion criteria
Randomized controlled trials (RCTs) and retrospective studies that comparing TaTME with LaTME were included. All the included studies had to have at least one of the relevant outcomes mentioned below. The exclusion criteria were as follows: (a) lack of the sufficient data or outcomes of interest; (b) duplicate publication; (c) non-comparative studies, editorials, letters, conference abstracts, review articles, case reports and animal experimental studies; (d) studies included high rectal cancer (tumor distance from anal verge more than 10 cm) and abdomino-perineal resection (APR).
Data extraction and outcomes of interest
Two independent authors extracted and summarized the data from the included studies independently.
The intraoperative outcomes were estimated blood loss, operative time, conversion rate, and intraoperative complications. The postoperative outcomes were overall postoperative complications, anastomotic leakage, ileus, urinary morbidity, reoperation, readmission rate, and length of hospital stay. The oncological outcomes were quality of mesorectum, circumferential resection margin (CRM), positive CRM, distal margin distance (DRM), positive DRM, harvested lymph nodes and local recurrence.
Quality assessment
For continuous variables weighted mean differences (WMDs) were calculated. For dichotomous variables odds ratios (ORs) were calculated. For continuous data as median and range values, the means and standard deviations were calculated by the formula described by Liberati et al. [22].
Statistical analysis
Statistical heterogeneity between studies was assessed using the χ2 test with significance set at p < 0.10 [23]. A random effects model was used and funnel plots were used to evaluate publication bias. The Newcastle-Ottawa scale was used to evaluate the methodological quality of all the retrospective studies.
Statistical analyses were done using RevMan 5.3 software (Cochrane Collaboration, Oxford, UK).
Results
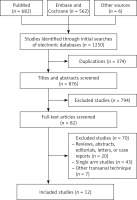
One thousand two hundred and forty-seven citations were retrieved from the search strategy. Finally, twelve studies [24–35] were included in the analysis, with a total of 899 patients (411 patients in TaTME group, 488 patients in LaTME group) (Figure 1). The characteristics of eligible studies are shown in Table I.
Table I
Characteristics of studies included in this meta-analysis
| Study | Design | Country | Patients TaTME/LaTME | BMI | T stage | Tumor location | Neoadjuvant therapy TaTME/LaTME | Quality score | |
|---|---|---|---|---|---|---|---|---|---|
| TaTME | LaTME | ||||||||
| Fernandez-Hevia 2014 [24] | MCC | Spain | 37/37 | 23.7 ±3.6 | 25.1 ±4.0 | T2–T4 | L + M | 28/23 | 7 |
| De’Angelis 2015 [25] | MCC | France | 32/32 | 25.2 ±3.5 | 24.5 ±3.2 | T2–T4 | L | 27/23 | 8 |
| Chen 2016 [26] | MCC | Taiwan, China | 50/100 | 24.2 ±3.7 | 24.6 ±3.1 | T2–T3 | L + M | 50/100 | 7 |
| Chouillard 2016 [27] | Prospective cohort study | France | 18/15 | 27.1 ±4.5 | 29.0 ±4.2 | T1–T3 | L + M | 14/12 | 7 |
| Lelong 2016 [28] | MCC | France | 34/38 | 24 (18.6–45.0) | 24.2 (17.7–32.7) | T1–T4 | L | 30/35 | 8 |
| Rasulov 2016 [29] | Prospective cohort study | Russia | 22/23 | 26.0 (19.7–32.3) | 26.0 (18.3–37.2) | T1–T4 | L + M | 19/11 | 8 |
| Chang 2017 [30] | MCC | Taiwan, China | 23/23 | 25.8 ±4.3 | 25.0 ±3.0 | T1–T3 | L | 8/14 | 7 |
| Mege 2018 [31] | MCC | France | 34/34 | 25 ±4 | 25 ±3 | T1–T4 | L | 29/29 | 8 |
| Persiani 2018 [32] | MCC | Italy | 46/46 | 25 (19.1–32.8) | 25.6 (18.8–33.4) | T1–T3 | L + M | 26/32 | 7 |
| Chen YT 2018 [33] | MCC | Taiwan, China | 39/64 | 25.4 ±4.0 | 24.6 ±3.3 | T1–T3 | L + M | 115/31 | 8 |
| Roodbeen 2018 [34] | MCC | Netherlands | 41/41 | 26.7 ±1.9 | 26.1 ±4.0 | T1–T4 | L | 18/18 | 7 |
| Rubinkiewicz 2018 [35] | MCC | Poland | 35/35 | 26.1 ±4.09 | 27.1 ±4.71 | T1–T3 | L | 31/31 | 7 |
Meta-analysis revealed no statistically significant difference in intraoperative outcomes: There were no statistically significant differences in blood loss (p = 0.85), operative time (p = 0.79), conversion rate (p = 0.69) or intraoperative complications (p = 0.70) between the two groups (Figure 2). There was no heterogeneity among studies, I 2 = 0%.
Figure 2
Forest plots describing estimated blood loss (A), conversion events (B), operative time (C) and intraoperative complications (D) between TaTME and LaTME

Ten studies [24–26, 28, 30–35] that assessed 821 patients reported on overall postoperative complication rate. Meta-analysis showed no statistically significant differences in overall postoperative complication (p = 0.39), anastomotic leakage (p = 0.60), ileus (p = 0.38) or urinary morbidity (p = 0.79) between the two groups (Figure 3). The TaTME group had non-significantly better postoperative outcomes in readmission (p = 0.08), reoperation (p = 0.34) and length of hospital stay (p = 0.09) (Figure 4).
Figure 3
Forest plots describing postoperative outcomes: overall postoperative complication (A), anastomotic leakage (B), ileus (C), urinary morbidity (D) between TaTME and LaTME

Figure 4
Forest plots describing postoperative outcomes: readmission (A), reoperation (B), length of hospital stay (C) between TaTME and LaTME

There were six studies [24–27, 31, 34] that reported CRM, eleven studies [24–26, 28–35] that reported positive CRM, eight studies [24–27, 30, 32–34] that reported DRM and five studies [25, 28, 31, 34, 35] that reported positive DRM. No differences were found in these pathological outcomes (Figure 5). Meanwhile, we did not find statistically significant differences in quality of mesorectum (p = 0.39), harvested lymph nodes (p = 0.62) or temporary stoma (p = 0.27) (Figure 6).
Figure 5
Forest plots describing oncological outcomes: CRM (A), positive CRM (B), DRM (C), positive DRM (D) between TaTME and LaTME

Figure 6
Forest plots describing oncological outcomes: quality of mesorectum (A), number of harvested lymph nodes (B) and temporary stoma (C) between TaTME and LaTME

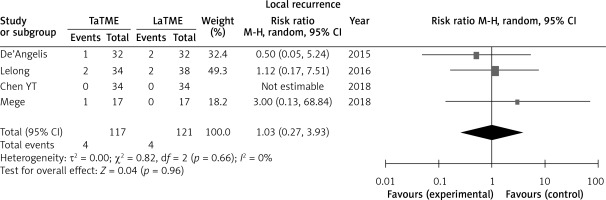
Four studies [25, 28, 31, 33] reported local recurrence; no difference was found in this outcome (Figure 7).
Publication bias
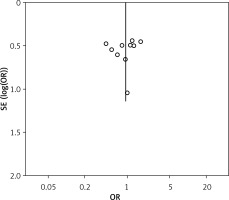
The funnel plot based on overall complication rate indicated no obvious publication bias (Figure 8).
Discussion
This study was the largest meta-analysis including 899 patients (411 patients in TaTME group, 488 patients in LaTME group). Our results showed no significant difference between TaTME and LaTME in overall intraoperative complications, postoperative outcomes, oncological outcomes or local recurrence. We hope that our findings can illustrate the safety and feasibility of TaTME, and promote its application in middle and low rectal cancer.
The TaTME is a novel technique which is expected to have better oncological outcomes. Lots of previous studies had shown that TaTME is superior to LaTME and may benefit in some surgical and pathological outcomes, but no RCT results prove these findings. There have been many meta-analyses [16–20] about TaTME in the last 3 years, but most of them contained substantial bias, and the results of Rubinkiewicz et al. [18] showed no significant differences in clinical outcomes between TaTME and LaTME recently. But we found this negative result based on overall complications and some surgical outcomes without systematically analyzing intraoperative outcomes, postoperative outcomes, oncological outcomes. What is more, TaTME is more suitable for middle and low rectal cancer, and it is inappropriate to include high rectal cancer, which was included in Rubinkiewicz’s study [35].
In this study, we included several of the most recent papers which were not included in previous analyses, and systematically analyzed surgical outcomes aiming to find out new proof of differences of clinical outcomes between TaTME and LaTME. Previous meta-analyses [16, 17] had conflicting results in conversion rate and postoperative complications. In this meta-analysis which included 899 patients, we were able to show evidence of decrease of the overall postoperative complication rate, urinary morbidity and readmission rate in the TaTME group. However, we found no significant difference in conversion rate in our result. As previous reports, temporary stoma may affect recovery after surgery [36–38], but there was no difference in the rate of temporary stoma between two groups.
In our study, the quality of mesorectum did not reach statistical significance between the two groups. A previous meta-analysis [16] including six studies found a significant difference in the complete rate of complete mesorectum. But after adding more studies in this study, no significant result was found in the complete rate of complete mesorectum. Interestingly, the TaTME group had better postoperative outcomes (readmission, reoperation, length of hospital stay) on average, although the difference did not reach statistical significance. The heterogeneities in these parameters were 10%, 15%, 76% respectively, which may be an important factor affecting these results.
Fewer studies have assessed long-term observation. Only Zhang’s meta-analysis [19] reported 2-year survival and 2-years disease-free survival between TaTME and LaTME, which included only two studies, and found no significant result. It is impossible to prove the superiority of any technique due to lack of new data. One of the most different procedures between TaTME and LaTME is separating the rectum during the small pelvis. Therefore, we think the rate of local recurrence is an important long-term outcome. In this study we first compared local recurrence between TaTME and LaTME; the result showed no difference between TaTME and LaTME. It means that changing this key operative approach may not affect the surgical outcome of TME. It still requires more time for long-term follow-up in RCT studies, or more any other long-term outcome data from non-RCT results.
This meta-analysis has several limitations that must be taken into account. Firstly, all the included studies were observational studies but without RCTs. Without adequate random sequence generation and blinding, the risk of bias might increase. Therefore, the quality of the evidence pooled from these retrospective trials must be judged as low. Secondly, there may be publication bias due to all the included studies being in English, and these data were not from a high-volume center, which may also affect the results. Finally, no long-term outcome, such as overall survival and disease free survival, was measured in the analysis.