Purpose
Artificial ascites is commonly used for the radiofrequency ablation (RFA) of hepatocellular carcinoma or liver metastasis locating in the periphery of liver, forming a space between target lesion and adjacent gut [1,2,3,4,5]. Inspired by such clinical applications of artificial ascites, our group previously has reported percutaneous artificial ascites injection to pelvic brachytherapy for the first time [6]. By this method, it was possible to create a space between gross tumor volume (GTV) and organs at risk (OARs) as well as to reduce the doses to OARs. However, trans-abdominal needle insertion under trans-abdominal ultrasonography guidance was not reliable, and the success rate was not high enough for daily clinical use because visualization of the tip of the needle in the abdominal cavity was unfavorable due to the presence of gas in the bowel. As such, there were cases where saline was only injected into the abdominal wall or mesenterium, not into the aimed peritoneal cavity.
Here, we describe a practical experience of novel transvaginal artificial ascites infusion (TVAAI) under trans-rectal ultrasonography (TRUS), which is an easier approach to distribute the artificial ascites precisely to the intended peritoneal cavity with better visualization than the trans-abdominal injection.
Case presentation
A 42-year-old female with a vaginal cuff recurrence was referred to our department for high-dose-rate interstitial brachytherapy. At the age of 35, she underwent simple hysterectomy for a cervical intraepithelial neoplasia. At the age of 42, a tumor in vaginal cuff was discovered by abnormal vaginal bleeding, and biopsy revealed small cell carcinoma. A whole-body physical examination, magnetic resonance imaging (MRI), and positron emission tomography (PET) showed that the disease invaded the left paravaginal tissue without reaching the pelvic wall. The greatest size of tumor was 34 mm, and there was no evidence of distant metastasis. The patient was initially treated with 4 courses of cisplatin (80 mg/m2) and etoposide (100 mg/m2). Consecutively, she received intensity-modulated radiation therapy with 45 Gy in 25 fractions to the whole pelvis, and boost irradiation with 24 Gy in 4 fractions of high-dose-rate interstitial brachytherapy (HDR-ISBT), using remote after loading system. HDR-ISBT was performed once per day in four different days, with TVAAI performed in every session. PET-MRI after chemotherapy (before radiotherapy) showed a remaining activity of the vaginal cuff tumor (Figure 1). At the time of first brachytherapy, the size of tumor was reduced to 26 mm. Figure 2 indicates the anatomical relationship of recurrent tumor and surrounding normal tissues in sagittal view of TRUS at the time of first brachytherapy.
Fig. 1
A) Axial, B) sagittal. The recurrent tumor (blue arrow) located above the vaginal cuff between the rectum and bladder on PET-MRI after chemotherapy. After hysterectomy, the intestine is located just above the tumor
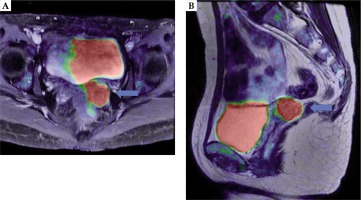
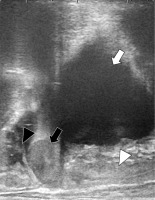
Fig. 2
Trans-rectal ultrasonography sagittal view before the first brachytherapy session. The recurrent tumor cranial to the vaginal cuff is next to the intestine. White arrow indicates the bladder, white arrowhead the vagina, black arrow the recurrent tumor, and black arrowhead the intestine, respectively
Transvaginal artificial ascites infusion and interstitial brachytherapy
Before TVAAI, we performed a hyaluronate gel injection (HGI) to the pararectal space and posterior paravaginal space according to a technique reported by Kishi et al. [7]. Hyaluronate gel was injected through vaginal cavity under real-time TRUS guidance [8,9,10]. After finishing HGI, the needle was further proceeded and penetrated the peritoneum through the pouch of Douglas, with a careful attention not to damage the intestine. Awareness in avoiding tumor puncture was also required in order not to induce iatrogenic implantation. After the operators confirmed that the tip of needle was in the abdominal cavity, a 500 ml injection of 5% glucose solution combined with 10 ml of contrast agent was performed, and it was shown that the liquid moved the intestine away (Figure 3). For TVAAI, no additional anesthesia or sedation was necessary. After finishing TVAAI, vaginal cylinder and interstitial plastic needle applicators into the tumor under TRUS guidance were inserted [11,12,13,14,15]. Subsequently, planning computed tomography (CT) was taken with a slice thickness of 2 mm generated by a large bore CT simulator (Aquilion, Canon, Tokyo, Japan) with patients in lithotomy position. Finally, brachytherapy dose optimization was performed to apply the prescribed dose to the GTV with a maximal sparing of OARs, using Oncentra brachytherapy planning software (Elekta, Veenendaal, The Netherlands). Figure 4 displays the positions of all applicators in relation to the GTV and OARs. Normal tissue single-fraction dose before and after TVAAI, which was calculated at the first session of HDR-ISBT is summarized in Table 1. With the introduction of artificial ascites, intestinal D2cc and sigmoid colon D2cc was reduced from 350 cGy/229 cGy to 0 cGy/84 cGy, respectively. On the other hand, the rectum and bladder dose did not differ significantly before and after TVAAI. TVAAI was performed four times in every HDR-ISBT session, with all applications resulting in a success.
Fig. 3
Trans-rectal ultrasonography sagittal view during the injection of 5% glucose to abdominal cavity through the pouch of Douglas. The recurrent tumor is invisible in this section. The intestine was pushed away by the artifi- cial ascites from the vaginal cuff. White arrow indicates the bladder, black arrowhead the intestine, white arrowhead hyaluronate gel between the vagina and rectum, and black arrow the needle penetrating the peritoneum, respectively. White asterisk shows artificial ascites in the abdominal cavity
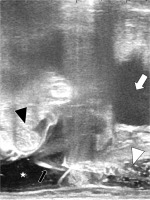
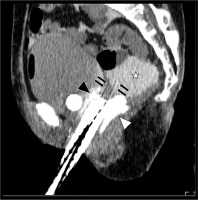
Fig. 4
Sagittal image of the planning CT after the procedures. White asterisk shows artificial ascites as 5% glucose with contrast enhancement agent, which lifted the intestine up from the tumor. Black and white arrowheads indicate hyaluronate gel spacing between the bladder and rectum, and black arrows show plastic interstitial brachytherapy needles penetrating the tumor. The artificial ascites and hyaluronate gel created distances between organ at risks and interstitial brachytherapy applicators
Discussion
Compared with percutaneous trans-abdominal injection of artificial ascites, TVAAI enables to infuse artificial ascites with better visualization of the needle tip as well as the injected fluid itself into targeted area. Additionally, HGI and TVAAI can be performed effortlessly, with the same needle without additional anesthesia or puncture.
There are spacer gels, which can be inserted into pararectal space to create an area between the rectum and prostate or vagina [9,10,16]. However, there are no agents, which can be injected into the peritoneal cavity, creating a space between vaginal stump of recurrent tumor and bowel adjacent to vaginal stump. TVAAI plays an important role as a spacer between the target volume and bowel floating in the peritoneal cavity using inexpensive materials. Although the intestinal dose could be 350 cGy without the artificial ascites, TVAAI effectively reduced the dose to the intestinal while keeping the tumor dose sufficiently elevated. This technique is most appropriate in a re-irradiation setting, where surrounding intestines already receive certain doses of irradiation.
The route of inserting a needle, in which no tumor exists between the puncture site and peritoneal cavity should be carefully planned beforehand, in order not to create iatrogenic implantation into the peritoneal cavity. If such a route exists, TVAAI can be applied for tumors with paravaginal invasion. So far, the actual rate of iatrogenic implantation related to TVAAI with needle penetrating tumor is not known and should be investigated in future researches. However, the authors believe that a dangerous needle pathway penetrating the tumor should be avoided when iatrogenic implantation is theoretically considered. TVAAI can also be applied for more common histology, such as squamous cell carcinoma or adenocarcinoma, as it happened in our case with small cell carcinoma.
The type of liquid, which should be used for artificial ascites for brachytherapy is not yet decided. In our previous report [6], we used saline. In contrast, when artificial ascites is used in RFA for liver tumors, 5% glucose solution is preferred because saline contains electrolytes, which provides a current. Moreover, the temperature of surrounding normal tissues around the treated site by RFA is higher with saline than that of 5% glucose solution, which does not passes the current [17]. Therefore, in brachytherapy, where unlike RFA, no high level of heat is created, both saline and 5% glucose solution are considered to be feasible. It is possible that saline would be preferred to 5% glucose solution in gynecologic brachytherapy to avoid infection where needles are inserted in the perineum, which includes the excretory organs. Especially for thin patients, if no contrast enhancement agent was used, it is difficult to distinguish artificial ascites from the bowel. Therefore, the authors recommend using contrast enhancement agent (if not contraindicated) to obtain better visualization.
As shown in this case report, repeated TVAAI is feasible with no severe complication related to this technique, supposedly because of no dangerous anatomic structures around the pouch of Douglas. However, because this novel technique is quite new, further experience with regard to toxicity is required.
There are several issues for TVAAI that should be considered. First, in case of tumor adhesion to bowel, even if artificial ascites is injected properly, no space between them is achievable. The presence of adhesion can be easily evaluated by TRUS: if the bowel next to the tumor moves smoothly, there is a doubtful presence of adhesion. Secondly, the time of fluid remaining in the peritoneal cavity is not known and should be investigated in further studies. Thirdly, complications or adverse effects related to TVAAI need to be additionally explored, including the rate of peritoneal dissemination, iatrogenic implantation, bowel injury, bleeding, or infections.
In conclusion, this report presented the easiness and effectiveness of TVAAI, which introduces an artificial ascites to the peritoneal cavity through the pouch of Douglas. This methodology was useful to create a space between the tumor target and OARs, such as the intestine, especially in the vaginal stump brachytherapy after hysterectomy. Further research is needed to investigate how the incidence of late radiation adverse effects for gastrointestinal tracts can be reduced by TVAAI.
Declarations
Ethics approval and consent to participate were acquired. Moreover, a written informed consent from patients for artificial ascites injection and brachytherapy were obtained. This case report was approved by the Institutional Review Board of the National Cancer Center Hospital (approval number, 2017-091) according to the ethical standards of the Declaration of Helsinki.
A written informed consent from the patient for publication, including each clinical datum as images, case history, and other clinical data was acquired.



