Purpose
Pancreatic cancer is the fourth most fatal cancer in both women and men, with a life expectancy of 2-5% at 5 years [1,2]. Moreover, most patients have already progressed to an advanced or metastatic stage of the disease at the time of diagnosis. Several preclinical studies established that pancreatic ductal adenocarcinoma (PDAC) is a systemic disease from the outset, displaying early micrometastatic spread [3,4]. Autopsy studies of primary resected PDAC patients showed that 70-85% of patients die of systemic recurrence rather than local disease [5].
Only 10-15% of all patients are eligible for surgery, which is presently considered to be the only potentially curative approach [6,7]. Even after surgery, PDAC remains highly lethal, as many patients develop hepatic metastases. Resectability status mainly depends on peripancreatic vessel contact/infiltration and presence or absence of distant metastases [8]. However, many PDAC patients undergo surgery of the primary tumor at some point and consequently have a biliodigestive anastomosis (BDA), which ultimately results in bacterial colonization of the intrahepatic bile ducts and complicating any hepatic metastasis treatment [9].
Treatment of PDAC metastases is challenging, since partial hepatectomy as a principal method not only failed to show any promising results, but also cannot usually be performed repeatedly due to impairment of liver function and the patient’s general condition [10,11]. Alternative measures like thermal liver ablation (RFA, laser therapy), are often complicated by cholestasis, bile duct strictures, and hepatic abscesses, with even higher incidence rates after previously performed BDA [12]. Even though being minimal invasive, thermal ablation measures underlie several restrictions such as tumor size (< 5 cm), heat sink effect, and inability to be used near thermosensitive structures.
In contrast to the aforementioned therapies, image-guided high-dose-rate brachytherapy (iBT) presents a different, anti-neoplastic, transcutaneous, and minimally invasive treatment option, and is applied in this study. Its efficacy and ability to provide local tumor control (LTC) has been proven by several investigators for different tumor entities in the past, achieving excellent local tumor control rates around 90% [13,14,15,16]. iBT is an afterloading technique that employs a 192Ir source, which is placed temporarily into the clinical target volume, i.e. the tumor. High-dose-rate irradiation is applied in a single fraction, providing an extensive cytotoxic effect via DNA and RNA damage to eradicate vital tumor cells. Other researchers examined the use of iBT for the treatment of patients with PDAC liver metastases and demonstrated a high local tumor control rate of 91% [17].
The goal of our study was to assess the efficacy and safety of iBT as a salvage maneuver for the treatment of liver metastases originating from PDAC.
Material and methods
Patient characteristics
Sixteen patients, with histologically proven PDACs and a cumulative number of 45 unresectable liver metastases, received treatment with iBT in our department between February 2010 and March 2017, and were enrolled in this retrospective study. Every patient was in a metastatic and progressive stage of disease at the time of referral to our department. Our study was approved by the local ethics committee.
Study design and eligibility criteria
Local tumor control (LTC) and overall safety of iBT were the primary endpoints of this retrospective study.
Each individual PDAC patient’s case was discussed at an interdisciplinary board of oncologists, interventional radiologists, radiation oncologists, and visceral surgeons who determined the indication for iBT for each patient individually.
The inclusion criteria were: 1) Resection impossible or unfavorable due to perioperative risk or loss of liver function; 2) Patient unwilling to undergo surgery, 3) Oligometastatic (≤ 5 metastases upon initial presentation)/controllable disease extent; 4) Adequate coagulation parameters (thrombocytes > 50000/nl, prothrombin > 50%, partial thromboplastin time < 50 s). Exclusion criteria were correspondingly: 1) Lack of consent, and 2) Uncontrollable tumor spread.
Interventional technique and irradiation
Prior to the iBT procedure, a whole-body contrast-enhanced CT and a Gb-EOB-DTPA-enhanced liver MRI (Primovist, Bayer Pharma, Leverkusen, Germany) were acquired for treatment planning and staging purposes. Physical status and laboratory parameters were also evaluated.
During and prior to the intervention, analgesia (fentanyl), sedation (midazolam), and local anesthesia (lidocaine) were administered. An 18-gauge needle was used under CT fluoroscopic guidance (Toshiba, Aquilion, Japan) or real time 1.0 Tesla MRI (Panorama 1.0T, open MR system, Philips Healthcare) to puncture the target lesions. In a next step, a flexible 6-French catheter sheath (Radifocus, TerumoTM) was placed using Seldinger technique over a stiff angiography guidewire (Amplatz, Boston Scientific, Marlborough, USA). Finally, the 6-French afterloading catheter (Afterloadingkatheter, Primed Medizintechnik Gmbh, Halberstadt, Germany) was introduced and the catheter ending temporarily fixated to the skin with sterile bandages and a cutaneous suture. Target lesion size and nearby structures at risk determined the number of catheters and their angulation. A CT scan in breath-holding technique or a Gadolinium-enhanced MRI were acquired for further treatment and irradiation planning as well as for catheter positioning confirmation. The interventional radiologist and the radiotherapist marked the clinical target volume and the adjacent organs at risk in every CT or MRI slice.
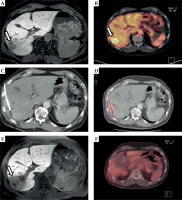
The irradiation design was devised employing the acquired dataset and the software system Oncentra (Nucletron, Elekta Ab, Stockholm, Sweden). The software was a part of the HDR afterloading system. The three-dimensional coordinates (x, y, z) of each positioned catheter’s tip in relation to the tumor margins were transferred into the treatment planning system. Furthermore, the calculated isodose lines were inspected in every imaging slice and adapted to the target lesion margins. An imaging example of the interventional technique is illustrated in Figure 1.
Fig. 1
Local tumor control in a patient with metastatic PDAC. A) Axial T1w Gd-EOB-DTPA (Primovist)-enhanced MRI (baseline MRI prior to iBT), arrow points to liver metastases; B) FDG-PET-CT demonstrates the activity of the hepatic lesions (arrow) prior to iBT; C) Inserted brachytherapy catheter in the liver lesions (white arrow) during CT-guided iBT; D) Colored lines represent the irradiation isodoses, with red line showing 20 Gy; E) Axial T1w Gd-EOB-DTPA-enhanced follow-up MRI after iBT with Gd-EOB-DPTA enhancement defect following irradiation; F) FDG-PET-CT (follow-up) shows no activity in the hepatic ablation area
The afterloading/iBT system (Nucletron, Elekta Ab, Stockholm, Sweden) applied an 192Ir source with a nominal activity of 10 Ci (370GBq). The irradiation was administered in a single fraction. The reference dose was defined as 20 Gy to enclose the entire target lesion (D99.9%); even higher, exponentially increasing doses were applied at the target lesion’s irradiation center. Prevention of new peripheral tumor incidences was achieved through implementation of a 5-millimeter security margin around the target lesion, i.e., the clinical target volume (CTV). Adjacent organs at risk such as the gastrointestinal tract (GI) were respected, and the irradiation scheme and dose correspondingly adjusted (empiric GI tract dose < 14 Gy/ml) [18].
Upon completion of the iBT procedure, the catheters were removed. The puncture sites were sealed by injection of gelfoam or fibrin tissue glue.
Follow-up
Response to iBT treatment was evaluated every three months after the ablation procedure: a Gb-EOB-DTPA-enhanced liver MRI, a contrast-enhanced CT (thorax and abdomen), clinical and laboratory evaluations were performed. Changes in size and enhancement defects were correlated in a dynamic T1w GRE sequence, DWI/ADC, post-Gd-EOB-DTPA, and a T2w sequence. Tumor edema was visualized in a T2w sequence, vital tumor tissue in DWI and late enhancement (post-radiation) defects in the post-Gd-EOB-DTPA sequence and the contrast agent dynamic sequences. Measurements were ultimately made in axial slices of the post-Gd-EOB-DTPA sequence in correlation with the DWI to account for vital tumor tissue and to differentiate from late enhancement defects. In some cases, an FDG-PET-CT was acquired.
Adverse events were recorded and defined corresponding to the Common Terminology for Adverse Events (CTCAE), version 4.03.
Local tumor control (LTC) after brachytherapy was defined corresponding to the Response Evaluation Criteria in Solid Tumors (RECIST 1.1) categories as stable disease (SD), partial remission (PR), and complete remission (CR). Progressive disease was defined as an increase of tumor diameter > 20% during follow-up.
Statistical methods
The primary objectives of this retrospective, single arm study were local tumor control and the overall safety of iBT. Progression-free survival and overall survival were secondary objectives. Calculations of LTC, PFS, and OS were done using Kaplan-Meier method with SPSS version 22 (SPSS, version 22.0; SPSS, Chicago Illinois).
Results
Sixteen patients with histologically proven PDAC, having a cumulative overall amount of 45 liver metastases, were treated with iBT in our department between 2010 and 2017, and were included in this retrospective study (Table 1). The median patient age at the time of diagnosis was 62 years (range, 35-73 years). Localization of the pancreatic primary tumor was as follows: nine in the head, six in the tail, and one in the body. Fourteen patients had a resection of the primary tumor prior to iBT: eight cases of Whipple/PPPD procedure and six cases of distal pancreatectomy. One patient’s primary was treated with iBT instead of surgery. Only one patient neither had resection nor iBT of the primary.
Table 1
Patients characteristics
| Total number of patients | 16 |
| Sex | |
| Men | 10 |
| Women | 6 |
| Age at time of diagnosis | |
| Median | 62 (Q1 = 55, Q3 = 69)1 |
| Range | 35-73 |
| Primary localization | 16 |
| Caput (head) | 9 |
| Cauda (tail) | 6 |
| Corpus (body) | 1 |
| Chemotherapy (before iBT)2 | 16 |
| Resection of the primary (before iBT) | 14/16 |
| Whipple & PPPD | 8 |
| Distal pancreatectomy | 6 |
| Other therapies | |
| Partial hepatectomy & radiation | 1 |
| SIRT | 1 |
| IBT primary (no resection) | 1 |
| ERCP (caput primary) | 3 |
| Metastases (cumulative) | 45 |
| Liver | 45 |
| Type of metastatic spread | |
| Synchronous | 5 |
| Metachronous | 11 |
| Lesion size (max diameter in cm) | |
| Median | 2.2 (Q1 = 1.3, Q3 = 3.3) |
| Range | 1-11.2 |
| Irradiation dose (iBT) (Gy) | |
| Median | 21 (Q1 = 17, Q3 = 24) |
| Range | 5-29.1 |
| Irradiation time (iBT) (min) | |
| Median | 29.8 (Q1 = 13.7, Q3 = 38.3) |
| Range | 8-82.8 |
| Number of catheters/lesion | |
| Median | 1 (Q1 = 1, Q3 = 2) |
| Range | 1-6 |
| Local tumor control | 39/45 (86.7%) |
| Local tumor control time (months) | |
| Median | 3.3 (Q1 = 2.8, Q3 = 5.5) |
| Range | 1.5-27.9 |
| Progression-free survival (months) | |
| Median | 3.4 (Q1 = 2.8, Q3 = 6.5) |
| Range | 1.5-19.6 |
| Overall survival after iBT (months) | |
| Median | 8.9 (Q1 = 5.6, Q3 = 8.9) |
| Range | 3.1-29.3 |
| OS from time of diagnosis (months) | |
| Median | 27.5 (Q1 = 19.5, Q3 = 51.3) |
| Range | 13-63 |
| Previous treatment (before iBT) | |
| Chemotherapy | 16 (100%) |
| Resection | 14 (87.5%) |
| Selective internal radiotherapy | 1 |
| IBT primary (no resection) | 1 |
| IBT image guidance | |
| CT | 26 |
| MRI | 19 |
| Time of hospitalization (days) | |
| Median | 4 |
| Range | 3-6 |
Synchronous metastatic spread was observed in five patients and metachronous spread in eleven patients.
Every patient received some form of palliative chemotherapy and showed disease progression prior to iBT: gemcitabine was administered to twelve patients and FOLFIRINOX to four patients. Gemcitabine monotherapy was amended in some cases: two cases of additional erlotinib, three cases of additional paclitaxel and two cases of additional oxaliplatin.
In the recent follow-up staging CT before referral to our department, every patient’s PDAC disease was found to be progressive under palliative chemotherapy; hence, iBT was applied as a salvage maneuver and chemotherapy discontinued four weeks prior to the iBT procedure. Disease progression was the primary reason for chemotherapy cancellation and drug-related toxicity was the secondary reason. Some patients received repeated iBT treatments, either to split the treatment and irradiation burden into two or more sessions, or to treat newly developed metastases later. A more detailed overview of the performed iBT and the dose applied is presented in Table 2.
Table 2
Organs at risk and tumor dose overview
[i] Table 2 shows 5 Gy liver volume %, the organs at risk (OARs) dose (Gy/ml), the tumor doses D90, D99.9, and Dmean (Gy) in median and range. A cumulative number of 26 brachytherapy interventions were performed. The n = … states the number of interventions were each organ was at risk, e.g. gastr n = 7 – gastric organ at risk in 7 out of 26 interventions (in 19 interventions D1 cc of 0 Gy/ml)
Treatment characteristics
The median tumor diameter was 2.2 cm (range, 1-11.2 cm). The number of inserted catheters per lesion during iBT varied between one and six, with a median of one. CT guidance was used in 26 interventions, MRI guidance in 19. The minimal planned/anticipated tumor enclosing dose was 20 Gy (D99.9%), which had to be adapted in some cases due to risk structures in proximity – a median irradiation dose of 21 Gy (range, 5-29.1 Gy) was administered. The median total irradiation time was 29.8 minutes (range, 8-82.8 minutes).
The intended tumor enclosing dose (D99.9%) was reached in 35 of 45 (77.7%) of all treated metastases. For the treatment of the other 10 lesions, the dose had to be adjusted due to risk structures in proximity.
The time of hospitalization ranged between three and six days, with a median of four days. Three patients developed a liver abscess (CTCAE grade 3) following an iBT session, which was successfully resolved with transcutaneous drainage and antibiotics, each without significant hospitalization prolongation. Four other patients received prophylactic periinterventional antibiotics as a precaution due to pre-existing, considerable cholestasis; no sign of infection or liver abscess was observed.
Local tumor control, overall survival, progression free survival
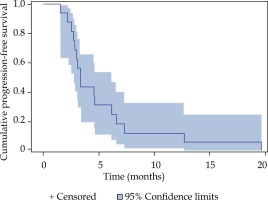
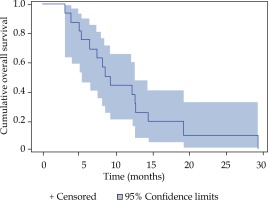
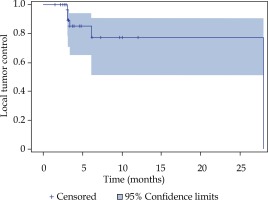
Local tumor control was achieved in 87% of all treated lesions in the Kaplan-Meier analysis (Figure 2). A cumulative number of six local relapses (liver metastases) were observed in four patients; one patient had a relapse of every treated lesion (three in total). The median progression-free survival (PFS) was 3.4 months (Figure 3). The median overall survival of the 16 patients with metastatic PDAC, calculated from the time of iBT was 8.9 months (Figure 4). The median OS from the time of PDAC diagnosis was 27.5 months.
Fig. 2
Local tumor control (LTC) after iBT of pancreatic ductal adenocarcinoma (PDAC) metastases, estimated with the Kaplan Meier method
Discussion
PDAC mortality rates and OS remain poor and have barely improved in the last decades; median overall survival (mOS) across various studies is still less than two years, mOS of metastatic PDAC is about 6 months [19]. However, two RCTs established recent breakthroughs in first line chemotherapy with FOLFIRINOX (OS, 11.1 months) and the combination of gemcitabine and nab-paclitaxel (OS, 8.5 months) compared with the traditional gemcitabine monotherapy (OS, 6.8 months) [20,21]. The downside of these “new” first line regimens is their limited applicability. The administration is reasonable only to patients with an ECOG PS of 0 or 1, which is the case for about 10-15% of all PDAC patients. In our study, four patients (25%) initially had an adequate ECOG PS and received FOLFIRINOX. An ECOG PS of 2 limits the options to gemcitabine monotherapy according to guidelines, which was the case for other 12 patients in our study.
Results from randomized trials comparing chemotherapy with chemotherapy plus conventional external radiation indicate no significant survival improvement with additional radiation [22].
The current standard of care for early-stage disease is surgery followed by adjuvant chemotherapy. Although surgical resection is widely considered curative, observed outcomes across various studies fail to support that presumption. A large analysis of more than 300,000 patients from the National Cancer Database demonstrated that mOS after resection of the primary was only 13 months [23]. Other large RCTs and trials support that data: mOS was less than 2 years [19,24,25]. The 30-day mortality after PDAC resection was up to 9% [19,24].
No treatment consensus exists regarding metastatic PDAC, which is considered unresectable based on the NCCN guidelines, apart from systemic therapy. While surgical metastasectomy being the standard method of choice for other cancer entities like colorectal cancer (CRC) or neuroendocrine liver metastases, the surgical approach fails to provide comparable promising results for PDAC metastases. The oncological value of liver surgery in PDAC patients is still highly questionable. Therefore, synchronous pancreatic and liver resections are only performed in very few PDAC cases, even in high-volume centers. A small study examined the outcome of seven patients after liver metastasectomy and published a mOS of 5.8 months [26]. Klempnauer et al. [26] reported a mOS of 8.3 months after synchronous liver and pancreatic resection and 5.8 months after metachronous hepatic resection. Klein et al. [27] published a mOS in a study (n = 22) of PDAC patients with synchronous hepatic metastasis resection of 7.6 months after surgery. Gleisner et al. [28] reported a mOS of 6 months even among highly selected patients with a low-volume metastatic liver disease. No benefit in overall survival was found in an older study by Takada et al. [29].
In contrast, the OS of our study calculated after iBT was 8.9 months, which is quite remarkable considering that all our patients had no further therapeutic options and were progressing under palliative chemotherapy, which was cancelled four weeks prior to iBT procedures. Furthermore, many of our patients had an unfavorable ECOG PS of 2, which rendered any surgical approach impossible. Re-challenge with alternative systemic anti-neoplastic regimens was either prohibited by the overall clinical condition or failed. To our knowledge, there is no comparable study evaluating the OS after resection of PDAC liver metastases in a salvage situation.
Another treatment approach for PDAC liver metastases are minimal invasive treatments like radiofrequency ablation. Park et al. came to the conclusion that selected patients with single, small sized (< 2 cm) PDAC liver metastases gain a survival benefit through an application of RFA [30]. However, certain restrictions of thermal ablation methods like RFA limit its applicability; tumor size < 5 cm, heat sink effect adjacent to vessels, high tumor vascularization, and proximity to central bile duct are the most important limitations.
In contrast, these restrictions do not apply to iBT, no size limit or cooling effects concerning brachytherapy are known. IBT even surpasses the size limit of 6 cm of stereotactic body radiation therapy (SBRT) and seems to induce fewer cases of radiation-induced liver disease (RILD) [31]. An advantage of local ablation measures like iBT or RFA concerning liver metastases is that it can be performed repeatedly while preserving liver function. The issue of potential needle track metastasis was addressed specifically by radiation of the interventional access as a precaution.
The results of our study (mOS 8.9 months, PFS 3.4 months, LTC 87%) confirm the data published by Wieners et al. (mOS 8.6 months, PFS 3.2 months, LTC 91%) [17]. The observed complication rate is also similar; we report three major complications in 45 iBT procedures, whereas Wieners et al. also report three major complications in 49 iBT procedures – in both studies with hepatic abscesses. A crucial risk factor promoting liver abscess caused by ascending biliary infection based on bacterial colonization is a biliodigestive anastomosis (BDA) in patients with prior Whipple procedures. Correspondingly, the three cases with hepatic abscesses in our study, which were successfully resolved with drainage and antibiotics, also had a BDA following resection of the pancreatic head. According to literature, major adverse events (grade 3 and 4) after iBT are observed in about 3% of cases [32].
Despite the promising results, limitations of our study are the relatively small patient collective and the retrospective, single arm design. The outcome of our study, however, substantiates the findings of Wieners et al. suggesting that iBT might prolong OS in a metastatic setting, which generally implies dreadful prognosis. Further investigations in prospective RCTs are necessary to validate the results of our small, retrospective analysis. Until then, the current treatment rationale should be to identify eligible patients for local treatment options in combination with systemic chemotherapy to prolong survival.