Purpose
Uveal melanoma is the most frequent primary malignant intraocular tumor in adults, with an annual age-adjusted incidence of 5.1 cases per million [1]. The main clinical goals of brachytherapy are tumor control, eye preservation, maintenance of vision, and quality of life.
The workflow in choroidal melanoma interventional radiotherapy is typically articulated in five main issues: 1) Multidisciplinary tumor board: case presentation and treatment choice; 2) Treatment planning: plan calculation and preplan approval; 3) Source preparation: applicator loading and sterilization; 4) Surgery: plaque implantation, treatment; 5) Plaque removal [2,3,4,5].
Radiation oncologist typically defines clinical target volume (CTV) considering tumor thickness from B-scan sonography images. A safety margin extension of 1-2 mm in all directions surrounding the tumor base [6] that accounts for microscopic extension of the tumor as recommended by the American Brachytherapy Society (ABS) [7], is usually defined as the planning target volume (PTV) [8]. An extra margin can be added by the radiation oncologist in case of doubts in plaque localization or tumor delineation [9].
Plaques are typically positioned under the tumor, and a dose of 85 Gy is usually prescribed to the apex of the tumor as recommended by the ABS [7]. A dose rate of 0.60-1.05 Gy/h delivering a total dose in 3 to 10 consecutive days is suggested [10]. For very posterior localizations, the plaques may be customized with notches of gold rim to allow the placement adjacent to the optic nerve sheath in juxtapapillary tumors.
Calculations are based on the reports of the American Association of Physicists in Medicine Task Group No. 43 [10,11]. In modern brachytherapy planning systems, recently implemented model-based dose calculation algorithms includes tissues heterogeneities. However, any change in the prescribed dose must be carefully verified before the introduction of model-based system into clinical environment [12].
Some authors have developed methods for COMS plaque brachytherapy to select intra-plaque ring radionuclides and source strengths depending on an individual patient to deliver more conformal and more homogeneous tumor dose distributions rather than uniformly-loaded 125I plaque [13] or even modified plaque for iris melanoma [14]. In general, clinical optimization of eye plaque brachytherapy is limited to tumor coverage, consensus prescription dosage, and dose calculations to ocular structures [13].
The severity of disorders (retinopathy, maculopathy, cataract, neovascular glaucoma, and nerve atrophy) before brachytherapy mainly depend on the amount of incidental irradiation of the respective tissues and ocular structures radiosensitivity [15,16,17,18,19,20,21,22]. So, an optimization with reduction of side effects without a loss of local tumor control is essential [23].
The aim of this technical note is to present a series of observations regarding the dosimetry used at our institution in order to reduce the dose to organs at risk and to adjust the treatment time to the needs of the center.
Material and methods
The three-dimensional reconstruction and the dosimetry was performed by a computer system developed by Dr. Astrahan at the University of California (BEBIG plaque simulator, version 2.16) [23]. Calculations were based on the reports of the American Association of Physicists in Medicine Radiation Therapy Committee Task Group No. 43 [10,11] for iodine plaques. Plaque heterogeneity correction functions were incorporated into the treatment planning, and the dose collimation by the gold-alloy backing and the global attenuation factor with the effect of eye plaque seed carrier were also included [24].
In dosimetry planning using 125I seeds applicator, there are three main factors to be considered: distance to organs at risk, source strength of seeds, and geometry of plaque.
Results
Distance to organs at risk
The distance of the applicator to organs at risk is perhaps the most important factor, since the dose presents a diminishment proportionally to the inverse of the square of the distance. Thus, the appropriate distance without sacrificing the tumor margins is able to protect (to a certain extent) the organ at risk. Although the plaques are typically placed under the tumor, it is permissible to slightly change their position in order to avoid critical structures (macula or optic nerve) without compromising the correct coverage of the tumor (Table 1) [25,26,27].
Table 1
Treatments features from our institution and other series of patients treated with episcleral brachytherapy, mean values. Seed Amersham model 6711 and Bebig model I25.S16 are used for ROPES and COMS plaques, respectively
| Shields [25] N = 1300 | Melia [26] N = 623 | Gündüz [27] N = 630 | Our institution N = 247 | |
|---|---|---|---|---|
| Radionuclide | 125I | 125I | 106Ru | 125I |
| Treatment time (h) | 120.6 | 141.2 | 120.0 | 129.50 |
| Dose rate (cGy/h) | 70.5 | 80.0 | 80.0 | 69.5 |
| Dose to tumor apex (Gy) | 85.0 | 95.2 | 91.2 | 85.31 |
| Dose to optic nerve (Gy) | 52.1 | 70.6 | 59.0 | 36.30 |
| Dose to lens (Gy) | 15.6 | 24.1 | 11.7 | 19.36 |
| Dose to foveola/macula (Gy) | 79.0 | 86.9 | 122.1 | 51.8 |
| Dose to sclera (Gy)* | – | – | – | 294.15 |
Mixture of sources
A mixture of sources of different source strengths can optimize the distribution of isodose and protect organs without sacrificing margins of the tumor (Table 1). Similarly, due to limited availability of an operating room (i.e., twice a week, only in the morning, etc.), the use of seeds with different reference of air kerma strength (RAKR) can modify the treatment time to adjust to accessibility of operating room. It does not influence the correct coverage of the tumor.
Finally, for mushroom-shaped tumors with much bigger height than the base, an optimal strategy is to load the plate with seeds of higher source strength in the center and with seeds of fewer source strength at the edges of tumor.
Geometry of the applicator
For each 125I seed, the geometry function in dose calculation formalism provides an inverse square-law correction based on approximate model of the spatial distribution of activity in the source, neglecting scattering and attenuation. As in anisotropy, the sources may be oriented in such a way that their ends are directed at the organ at risk, since the self-absorption of the source is notable at these points and the dose falloff can be remarkable.
The seed carriers of the plaques can be manually rotated within the shell, so that a roughly elliptically shaped subset of the seed positions in the carrier would spatially conform to the shape of the tumor base and margin without over irradiating organs at risk (Table 1). The plaques can also be rotated to balance the distance of the suture eyelets from the limbus to simplify the surgery. In investigated case, the dosimetric differences were moderate during rotation of the seed carrier.
Discussion
In the study (Figure 1), a reduction of dose to the macula and optic disk was observed from 62 Gy and 52 Gy, to 45 Gy and 40 Gy, respectively, while maintaining dosimetric coverage of the tumor. The optimization of treatment time has an immediate effect on availability of operating rooms at hours that are convenient for our institution, and this is important circumstance from the logistic point of view.
Fig. 1
Different plans according to the points of improvement. Tumor with a circular size of 11 mm of diameter and 5 mm of height is presented. The COMS plaque diameter is 16 mm. The first row is a plan without any consideration. Dose to critical structures and treatment time (operating room is available preferably in the mornings) clearly improving. Next row shows a big improvement by separating the plaque from organs at risk. Next row presents a mixture of seeds to adjust the treatment time. Next row shows how the seed carrier rotates into the plaque with a little improvement of the dose to macula. The last row is the final plan of treatment; it is a combination of all techniques described. The time of insertion was always the same: Monday 21/01/2019 at 9:30 am
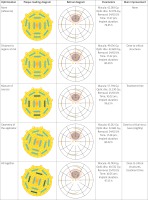
It is worth to mention that the techniques were used previously at our institution, and there was no difference in either both local tumor control [4] or complications [3,5]. Moreover, for many side effects, the results were better for patients treated at our institution [3,4,5]. Table 1 shows a systematic reduction of the dose to fovea and optic nerve in our institution compared with other centers and radionuclides.
At our institution, new seeds arrive with the frequency of two months. Once a new order is received, the oldest sources are discarded and only sources of less than 6 months old remain for use. From the therapeutic point of view, three sets of seeds are sufficient for an optimal personalized dosimetry. Therefore, the availability of different radionuclides and their possible combined use for 125I applicators shows a significant progress in dosimetry, as found in other studies [28,29,30].
According to our quality assurance protocol, the seed lots are stored in separate compartments. Before and after the assembly of the plaque, the RAKR of each seed is confirmed with a well counter. The verification of the correct source strengths and assembly is conducted by two dosimetrists.
Conclusions
In this paper, we present the different options for an optimization of episcleral brachytherapy regarding the doses and treatment plans. The implementation of these techniques can be of great support for an institution to optimize the resources of brachytherapy unit. The aforementioned strategies have been used at our institution with great success, and no decrease in local control nor an increase in toxicity have been noted.



