Introduction
Trigeminal neuralgia (TN) is a relatively uncommon chronic condition, affecting less than 0.5% of the general population [1]. It manifests itself as episodic attacks of sharp, electric, shock-like pain, usually unilateral, in the regions of the face subject to the fifth cranial nerve (CN V). Attacks are triggered by movements of the facial muscles, cold temperature, touch or are spontaneous in nature. TN is included in the 13th chapter in the International Headache Society classification [2]. Based on the aetiology, it is systematized there as classical (due to vascular nerve compression), secondary (evidence of clear cause) or idiopathic (no cause is apparent). Recently, TN has been noted as one of many possible neurological complications of coronavirus disease 2019 (COVID-19). Since December 2020, when COVID-19 vaccines became the primary form of pandemic control, about 13 billion doses of vaccines have been administered worldwide. Having done a literature review, we came across only a few cases of TN in total that were postulated to have developed after COVID-19 vaccination [3-6]. Herein we present a case of TN, which originated shortly after the 3rd dose Pfizer BioNTech COVID-19 vaccine, discussion on its differential diagnosis and suggested effective treatment, based on our observations.
Patient case presentation
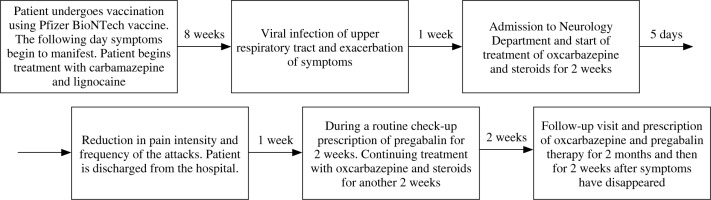
A 36-year-old woman was admitted to the Department of Neurology due to persistent pain in the left side of her face. The pain had first appeared two months ago, on the day following the vaccination using the third dose of the Pfizer BioNTech COVID-19 vaccine. Initially, the entirety of the left side of her face had been affected, but a few days later the pain became localized in the region of the second and third branch of the left trigeminal nerve. The pain was paroxysmal, presenting itself as attacks lasting 4-5 seconds each, triggered by movements of the mouth and jaw during activities such as eating or brushing the teeth. Attacks had been excruciatingly strong, with a score of 10 according to the numerical rating scale (NRS), and as such prevented the patient from participating in everyday functions, finally causing her to seek help at the Emergency Ward. Allodynia with painful sensation after application of cold air and hypersensitivity to stimuli were prominent during preliminary examination. Patient reported subjective, distorted sensations in the region of the second branch of the left CN V during exacerbations of pain, but no objective changes in the sense of touch examination were found. No other complaints or changes were observed in neurological evaluation. No relevant history of previous or concomitant diseases was reported. No familial history of neuralgia or similar conditions was reported by the patient. The previous two doses of the same type of vaccine were taken without any complications or adverse effects.
Standard laboratory tests showed no abnormal results. Concurrent COVID (the real-time quantitative polymerase chain reaction [RT-qPCR] test displayed negative results), as well as other potential ongoing infections, were excluded. There was no elevation in D-dimer levels, strongly suggesting a lack of pathological thrombotic processes. Magnetic resonance imaging (MRI) of the head with contrast did not show any significant pathologies that could contribute to the development of symptoms.
Prehospital, initial treatment consisted of carbamazepine in the dose of 200 mg taken twice a day, and Lignocainum hydrochloridum 5 mg per kg of body mass, used to alleviate stronger attacks. This management strategy was only partially effective and caused a decrease in frequency and intensity of pain paroxysms, but resulted in multiple side effects, mainly somnolence, which significantly impacted the daily living of the patient.
Sudden exacerbation of pain was observed after about eight weeks of the treatment described above and was associated by the patient with the onset of concomitant viral infection of the upper respiratory tract. After admission to the Neurological Department, a second MRI with angio- graphy (MRA) was performed (according the European Academy of Neurology guidelines 2019) [7], which revealed no neurovascular conflict.
The new treatment was instituted with replacement of carbamazepine by oxcarbazepine 600 mg twice a day and introducing steroids based on a previous scientific report [3] encountered during our research following the patient’s interview. Our case matched one description very well and, as such, with the patient’s cooperation, therapy based on the reported findings commenced. Steroids were given in the form of dexamethasone 12 mg per day for two weeks and titrating doses during the following two weeks. After combined therapy employing steroids with oxcarbazepine, a reduction both in pain intensity and in frequency of attacks was observed within five days. Because the pain was still present, the treatment was further supplemented with pregabalin in the dose of 150 mg per day for two weeks and was continued for the next two months. Gradually, but with transient periods of weak exacerbation, pain alleviation was achieved and the patient made a full recovery. Treatment with oxcarbazepine and pregabalin was continued for two further weeks. Six months after the onset of the disorder the patient remains without pain. Treatment was officially declared complete in May 2022 (Fig. 1).
Discussion of differential diagnoses
Differential diagnosis of TN includes possible presence of other similar conditions, such as glossopharyngeal neuralgia, cluster headaches, painful post traumatic trigeminal neuropathy, persistent idiopathic facial pain, herpes zoster related neuropathy and dental pathologies. Diagnosis relies on highly specific clinical features, allowing it to be easily distinguished from aforementioned ailments. Despite superficial similarities, such as with cluster headache symptoms overlapping somewhat with those of TN, based on different localizations, duration of the attacks and accompanying autonomic signs, a physician should be able to easily provide a proper diagnosis. What is more, in cluster headaches pain tends to migrate from one side of the face to the other, while it consistently remains limited to a single side in TN, usually in the 2nd or 3rd branch of CN V. Triggering factors other than those typically found in TN and different pain quality may indicate persistent idiopathic facial pain. Painful post-traumatic trigeminal neuropathy may resemble TN, but it is always preceded by a major traumatic injury and displays clear neurological abnormalities visible on neuroimaging. Herpes zoster neuropathy shares many common features with TN, but it is differentiated by the presence of highly distinctive skin lesions, usually in the 1st branch division of CN V. Altogether, using judicious observation and exclusions, a physician familiar with the basics of TN should be able to recognize it without much of a struggle.
Discussion of the final diagnosis
The patient described in this case report displayed a multitude of clinical features prompting us to diagnose her with a case of TN. These included specific triggering stimuli, that is cold, touch or movements of the jaw, characteristics of the pain, which was sharp, stabbing, and electric-like, affected area, limited to the 2nd branch of CN V unilaterally, and duration of the attacks, shorter than two minutes.
Differential diagnosis requires a thorough examination to classify TN according to specific subtype. We excluded the possibility of it being a classical TN based on the lack of evidence of neurovascular conflict in MRA. Secondary TN was excluded based on absence of space-occupying tumours, a demyelinating process or other disorders. These results leave us with the possibility of either TN idiopathic or TN attributed to other causes. However, the patient did not suffer from any other conditions predisposing her to develop TN.
There have been many case reports in the literature which concerned TN as a complication of COVID-19 infection [8, 9] and a few reports on TN developing after COVID-19 vaccination [3-6] as well. Neurological complications during the course of COVID-19 infection, apart from TN [8, 9], include the neuropathies facial nerve palsy [10], sixth cranial nerve palsy [11] and Guillain-Barré syndrome [12]. So far, four cases of TN after COVID-19 vaccine have been described, all of which occurred after the Pfizer-BioNtech vaccine, after either the first [3, 5, 6] or the second dose [4]. Our case is the first case of similar symptoms developing after the third dose of the vaccine.
The pain was variously accompanied by a multitude of other neurological symptoms, including numbness in the V1, V2 or V3 branches of CN V, cervical radiculitis [5] and numbness of the left upper limb [4]. Neuroimaging studies are usually recommended to distinguish classic TN from secondary TN [13], and such studies were performed in each case we have encountered. Changes in MRI were detected in two cases as abnormal asymmetric thickening and robust perineural sheath enhancement of the V3 segment of the left CN V [5] or as hyperintensity in the right lateral dorsal pons, at a level above the CN V origin [4]. However, in the remaining cases, including the one described here, neuroimaging showed no significant changes.
Treatment of TN depends on a number of factors, such as age, general health, severity of symptoms and the cause of the condition. The first-line treatment of idiopathic TN usually is restricted to pharmacotherapy [13]. In patients who developed TN after a vaccine, administration of steroids significantly reduced the pain frequency and intensity and improved patients’ condition [3-5]. Steroids were effective when administered both intravenously [3, 4] and orally [4, 5]. In one case combination of pregabalin and carbamazepine alone reduced pain and ameliorated facial numbness [6]. Pregabalin alone was insufficient to control pain attacks in all reviewed cases.
The immune-related reaction is suspected to be the underpinning pathomechanism in the described cases. Such a pathomechanism is proposed as a cause of neurological complications after defective immunization, with demyelination of the central nervous system (CNS) reported [4]. One of the currently suggested pathomechanisms of TN is local demyelination within the CN V root [14]. This process is usually triggered by compression of the root of the nerve, such as in the case of neurovascular conflict. Both peripheral and central demyelination was reported as a rare complication after COVID-19 vaccination [15].
In the cases presented so far in the literature and in our case, neuralgia symptoms developed within a few days, or sometimes even hours, after the vaccination [3-6]. The process that led to demyelination would have to develop rapidly. In addition, in the literature we can find dozens of cases of demyelination of the CNS, other than TN occurring after vaccination. It has been speculated that the mechanism for the development of this demyelination may be bystander activation [16]. Single-stranded mRNAs are able to activate TLR-7 and TLR-8 receptors, causing an increase in secretion of proinflammatory cytokines and a strong response from T and B lymphocytes, which results in activation of existing self-reactive T and B lymphocytes and development of inflammation [17]. The occurrence of this mechanism in the cases of TN discussed here is supported by the short intermediary period from the vaccination to the onset of symptoms. It is possible that the occurrence of such adverse reactions only in a relatively small number of vaccinated individuals is due to a genetic predisposition. Certain polymorphic variants of the pattern recognition receptor (PRR) may induce a stronger immune response [18]. Other mechanisms that can cause CNS demyelination include molecular mimicry and epitope spreading [16].
Our case is the first, as far as we know, occurrence of TN possibly occurring in relation to the 3rd dose of the vaccine. However, based on the data provided by the Centers for Disease Control and Prevention (CDC) [19, 20] we can conclude that the incidence rates of both local and systemic side effects after the 2nd and 3rd doses were comparable and only slightly higher than those occurring after the 1st dose. Remaining on the subject of vaccinations, there is some speculation that the stronger immune response after the non-first doses may be due to the differences in the immune environment encountered by those doses. In the case of non-first doses, the vaccine affects not only naïve cells, but also primary specific antibodies and memory T and B cells formed after the first dose. Additionally, prime-induced resting trained innate cells can respond better than naïve cells to restimulation [21]. The exact molecular mechanisms of immune memory formation and maintenance after vaccination are not fully elucidated, but circulating antibody levels provided by Pfizer- BioNTech COVID-19 vaccination are greatly reduced at 6-8 months after vaccination [22]. Since our patient was vaccinated each time using the Pfizer-BioNTech COVID-19 vaccine, at the constant dose, maintaining the typical interval and employing the same route of administration, we may speculate that the above-described phenomenon might have influenced the occurrence of the adverse reaction after the booster. It still remains unclear why TN developed after the 3rd rather than the 2nd dose, but we can suppose it might have been influenced by other independent factors, which could have caused exaggeration of immunological response such as a subclinical infection the patient might have been suffering from, in the period when the vaccination took place. It is also worth remembering that peak antibody levels are typically reached after three vaccine doses [22]. The exacerbation of pain which was observed later during the course of TN was triggered by viral pharyngitis, an event with immunological implications. The beneficial effect after steroid treatment may indicate, again, the excessive immunological response as a cause. The improvement could not be entirely due to simultaneously introduced oxcarbazepine, because previously management with carbamazepine was partially effective. Carbamazepine and oxcarbazepine share approximately the same mechanisms and clinical efficacy, but in our case, radical improvement after switching from the former to latter, with the addition of steroids, was not only due to the alleviation of adverse effects, but also better pain control. Possibly, administration of steroids could act causally.
It is important to note that the nerve damage would derive not from the direct actions of the virus, but rather from the exaggerated and disproportionate immunological response of the organism to it. As such, it follows that the virus itself is not necessary for the nerve damage to occur, with only improper reaction to it being indispensable. Thus, the COVID-19 mRNA vaccine, which does not contain the virus proper, but generates, by its design, a response resembling that to the virus, could possibly cause similar symptoms to present themselves if this response is similarly distorted.
As was mentioned before, diagnosis of TN relies almost exclusively on the patient’s history and symptoms reported and observed. Based on the clinical diagnosis, we cannot ascertain that there was an undisputed correlation between the occurrence of TN and vaccination. Neurovascular conflict was excluded, and so were the secondary causes, but it is exceedingly difficult to ensure that no idiopathic capacity was present. The question remains whether the origin of the disease was entirely spontaneous or the occurrence just coincided with the vaccination. The small number of similar cases does not allow us to confirm any categorical associations between the vaccine itself and the observed symptoms due to the lack of factual evidence supporting them, but, on the other hand, the infection itself has been definitively linked to multiple symptoms affecting the nervous system [23].
As can be seen in the Table 1, delineating the so-far reported cases of TN disorders possibly related to the COVID-19 vaccine, a number of those cases [4-6] presented themselves with additional sensory disturbances in the trigeminal nerve territory, absent in the other cases [3], including ours. Numbness and other sensory dysfunctions are not typically seen in TN, but rather in painful trigeminal neuropathy [2]. Painful trigeminal neuropathy is recognized as a separate condition, involving damage to the trigeminal nerve causing the loss of sensations. In TN proper, the nerve damage is less pronounced and causes increased function, rather than its loss [24]. We can assume that in those cases the sensory disturbances and the sensation of numbness could have been caused by the greater degree of damage done to the trigeminal nerve. It is also worth noting that those clinical features tended to be the most persistent ones, outlasting pain.
Table 1
Table comparing cases of trigeminal neuralgia with possible connection to COVID-19 vaccination reported to date
| Time of onset | Clinical presentation | Interventions used | Clinical outcome | |
|---|---|---|---|---|
| Case 1 Kaya and Kaya [3] | After 1st dose of vaccine | Typical trigeminal neuralgia, affecting the right side of the face, without any clear underlying pathologies | Initially pregabalin, later incidentally steroids | All symptoms subsided after the introduction of steroids |
| Case 2 Onoda et al. [6] | After 1st dose of vaccine; a month prior the patient had undergone surgery to alleviate already existing TN | An occurrence of trigeminal neuropathy of the right side of the face in a patient with prior trigeminal neuralgia treated surgically | Carbamazepine and pregabalin | Partial recovery, with persistent numbness in the affected area |
| Case 3 Lee et al. [4] | After 2nd dose of vaccine | Trigeminal neuralgia, with additional sense of numbness in the region of the left upper limb. MRI revealed lesions in the pons, in the surroundings of the entry of trigeminal nerve | Carbamazepine, concurrently with methylprednisolone, later prednisolone | Full recovery, patient forwent carbamazepine due to the efficacy of steroids |
| Case 4 Narasimhalu et al. [5] | After 1st dose of vaccine | Classified as trigeminal neuritis with radiculitis. Oedema with increase in the diameter of the perineural sheath was observed in the MRI | Pregabalin, later supplemented with methylprednisolone | Majority of the symptoms were alleviated after the use of prednisolone, with numbness persisting |
| Case 5 | October 2022, after 3rd dose of vaccine | Typical trigeminal neuralgia affecting the left side of the face, without any clear underlying pathologies | Initially carbamazepine and lignocaine, later oxcarbazepine, dexamethasone and pregabalin | Successful recovery of the patient after switching to the second line of therapy |
Conclusions
The temporal relationship, history and exclusion of other causes suggest that TN can occur in patients after the third dose of the Pfizer BioNTech COVID-19 vaccine. Therapy with steroids, oxcarbazepine and pregabalin may reduce the frequency and intensity of pain attacks of TN of such origin. Recurrences and exacerbations of pain are possible during treatment, as seen in our case. It cannot be ruled out that the association between the vaccine and TN was a coincidence and not a causal relationship, so further observation of subjects vaccinated against COVID-19 and investigation of the causes of TN must continue.
Take-away points
Information about COVID vaccinations should be included in the history of patients developing TN and should be considered as a possible cause of TN.
TN can occur as a complication even after subsequent doses of the COVID vaccine when other possible causes are ruled out.
Steroid therapy may be effective as a treatment in TN as a possible complication of COVID vaccination.
Patients with TN as a possible complication of COVID vaccination should preserve vigilance to avoid concomitant infections which can exacerbate symptoms.