Neurogenic tumors localized within the mediastinum are very rare and occur almost exclusively in the middle and posterior mediastinum. They tend to occur in older individuals (mean age: 46 years), predominantly in women [1, 2]. Patients are usually asymptomatic, or present with local malignancy (compression syndromes). About 50% of patients have arterial hypertension and other symptoms related to release of catecholamines by the tumor. If resection of the tumor is feasible, a 50% recurrence rate is reported; it metastases in about 25% of cases [3]. Very rare paragangliomas arise from cardiac tissue and are usually located subepicardially, at the roof of the left atrium. Here, we describe a case of paraganglioma in an 8-year-old girl located in the area of the left coronary artery origin and bifurcation. Prior to diagnosis, the symptoms of tumor had been misdiagnosed and treated as bronchial asthma.
An 8-year-old girl with a non-characteristic medical history of shortness of breath, fatigability, exertional dyspnea and tachycardia as well as chest tightness was referred to the family doctor. Symptoms occurred irregularly and were not always present after physical effort. The family doctor carried out physical examination and laboratory tests (blood gases, blood morphology). The results of physical examination, and basic biochemical and hematological tests were unremarkable, apart from mild anemia (Table I).
Table I
Results of blood gases and peripheral blood morphology
Screening echocardiography revealed good function of the ventricles and valves and no structural heart defect. Electrocardiography showed normal sinus rhythm with no characteristic changes. She was referred to the department of pediatric cardiology.
Based on initial symptoms and known clinical results the hypotheses of cardiac disease, respiratory problems and endocrine disturbances were taken into account.
Detailed echocardiography as well as magnetic resonance imaging (MRI), angio-computed tomography (angio-CT) scans and ultrasound examination of the neck and abdomen was carried out. Accessory biochemical tests were performed to differentiate clinical symptoms. These included: indicators of heart ischemia (CKMB, hsTnT), heart failure markers (NT-proBNP), markers of germinal tumors (AFP – α-fetoprotein, β-HCG) and urine catecholamine metabolites concentration (Tables II, III).
Table II
Results of serum laboratory tests with reference values
Table III
Results of urine laboratory test with reference values
Ultrasound of the thyroid gland showed normal echogenicity of the gland with a regular pattern of vascularity. The size of the thyroid gland was within normal limits. The lymph nodes of the submandibular area were slightly enlarged.
Ultrasonography of the abdomen demonstrated normal appearing liver without evidence of intrahepatic biliary dilatation. The gallbladder was normal in appearance without evidence of stones or wall thickening. The common bile duct was not abnormally distended. The head, body, and proximal tail of the pancreas were normal. The spleen was not enlarged. The kidneys were normal in size and echogenicity. No signs of collecting system dilatation were noted. There was no free fluid within the abdomen. The abdominal aorta and IVC were normal in caliber.
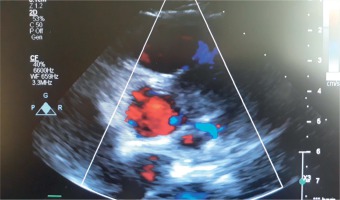
Detailed echocardiography revealed presence of a tumor in the area of the origin of the left main coronary artery and its division into the left anterior descendant and circumflex artery. The flow through left main, left anterior descending artery (LAD) and circumflex (Cx) was well preserved (Fig. 1). The involvement of the left coronary artery was visualized with the tumor mass surrounding the wall of the aorta and spreading towards the left ventricular free wall, alongside the left descending artery. The function of the left and right ventricle was normal. There was normal regional wall motion, normal right ventricular cavity size and systolic function, normal left atrium cavity size and no pericardial effusion.
Fig. 1
Echocardiogram of the heart with extrarenal paraganglioma localized at the left main coronary artery and its bifurcation to the LAD and Cx arteries. Arrow shows presentation of color Doppler scan of the flow in left main and bifurcation within the mass of the tumor
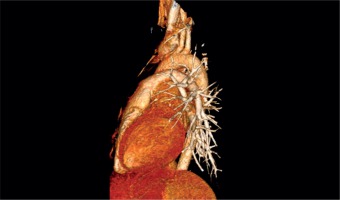
Chest MRI and angio-CT scans demonstrated a 4 × 3.8 cm mass of nodular structure and irregular density localized at the area of the ostium of the left main coronary artery, well separated and located outside of the heart cavities, subepicardially. It was located directly under the main pulmonary artery and attached to the ascending aorta including the left main and division of the left coronary artery into LAD and Cx branches (Fig. 2). The size of the tumor was 3.0 cm × 3.5 cm × 3.5 cm.
Fig. 2
Angio-CT of the thorax: 3D reconstruction of the heart and great vessels. The arrow indicates the localization of the tumor underneath the pulmonary trunk compressing the left main coronary artery, circumflex and left anterior descending artery
Because of the unclear clinical picture and atypical localization of the tumor, the patient was qualified for tumor biopsy for histological assessment. A limited sternotomy was performed and the tumor localized at the area of the orifice of the left main coronary artery was visualized. The oval shaped mass was dense, whitish at the surface and was located under the pericardium. Three small pieces of the tumor were carefully harvested and sent to the pathology department for histopathological assessment. During the harvesting procedure destabilization of the patient occurred. The pressure became high, and tachycardia was noted. Than a sudden drop of the blood pressure was noted with accompanying atrial flutter with atrio-ventricular block. The treatment of the hemodynamic disturbances was immediately initiated. The patient received an infusion of dopamine and the heart was synchronized by electrical cardioversion, which was successful at the third time.
The post-operative period was hemodynamically stable. On the second postoperative day, right pleural effusion was noted and drainage was applied.
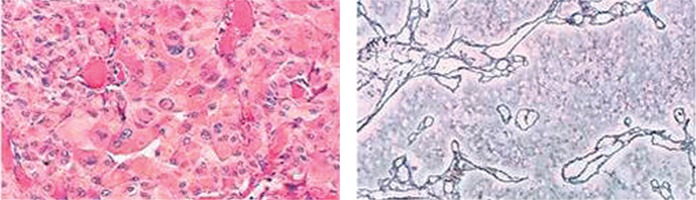
Histologic assessment of the tumor biopsies revealed extraadrenal paraganglioma. It showed a nesting pattern of cells associated with a prominent vasculature. Immunohistochemical staining revealed presence of CHA (+) cells, SYN (+) cells, EMA (–), and proliferative index Ki-67 about 5%. Numerous cells with hyperchromatic nuclei were visualized in the tissue of the tumor.
In the pericardial effusion mesothelial cells (calretinin+), lymphocytes, granulocytes, plasmocytes and macrophages (CD68+) were found. There was no neoplastic cells in the pericardial effusion (CHA+) (Fig. 3).
Fig. 3
Microscopic picture of the histologic specimens of the tumor biopsy. A nesting pattern of cells associated with a prominent vasculature was revealed. Immunohistochemical staining showed presence of CHA (+) cells, SYN (+) cells, EMA (–). Numerous cells with hyperchromatic nuclei were visualized in the tissue of the tumor
Echocardiography in the postoperative period showed good function of the left ventricle (FS 34%, EF 64%). The flow through the left coronary artery of 3 mm in diameter was patent.
Having the histologic assessment, perfusion scintigraphy scans were carried out to assess the metastatic potential of the tumor (Fig. 4).
Paraganglioma is a rare tumor stemming from neural crest cells. Intrapericardial tumors arise from ganglia associated with the aorta, pulmonary arteries or coronary arteries [4]. Extremely rare are primary paragangliomas arising from the heart and on average they occur in the fourth decade with slight prevalence in women.
Clinical symptoms depend on localization and functional status of the tumor and usually are grouped into local (related to local malignancy) and general symptoms. Patients with active tumors usually have symptoms related to catecholamine secretion: hypertension, cardiac palpitations, tachypnea, headaches, and sweating. Locally, if the tumor compresses systemic vessels, pulmonary vessels or coronary arteries, it causes relevant symptoms. In our 8-year old girl, both general and local symptoms suggested problems with the respiratory system (asthma), heart (rhythm disturbances) or an endocrine (thyroid gland or suprarenal) disorder. In the initial diagnostic work-up these three main directions were taken into consideration. Simultaneously biochemical tests (Tables I and II) and imaging (CT, ultrasonography, echocardiography) were carried out. Initial biochemical tests and negative results of abdomen, neck and chest ultrasound made the diagnosis difficult. Detailed echocardiography and angio-CT revealed the tumor localized at the base of the heart in the area of the left coronary artery orifice. Because of the unclear clinical picture, biopsy of the tumor was recommended. Although minimally invasive, the procedure was hazardous, because of dangerous destabilization of the patient apparently after release of catecholamines into the blood.
Intraoperative histopathologic assessment of the harvested cells revealed paraganglioma. Detailed immunohistochemistry confirmed the diagnosis. The only curative treatment of a paraganglioma is total resection. However, the results of the CT, MRI and coronarography (angio-CT), localization of the tumor and course after harvesting of the biopsy indicated non-operability of the tumor. Because of the invasive nature of the tumor, many patients require extensive reconstructions of the involved tissues and even auto-transplantation of the heart. A successful resection of the tumor without cardiopulmonary bypass was reported very rarely, and usually if the hormonal activity of the tumor was known, alpha blockade was used to prevent catecholamine-related complications [5, 6]. In our patient, the nature of the tumor before harvesting the biopsy was unknown. Pharmacologic prophylaxis was not undertaken and we faced considerable problems with hemodynamic stability. After conformation of the histopathology diagnosis, a b-blocker was used to prevent catecholamine-related hypertension and 24-hour blood pressure monitoring was carried out to control blood pressure of the patient. The dynamics of the tumor is very fast and partial resections are not curative. It reveals metastases very quickly. Taking it into consideration an metaiodobenzylguanidine (MIBG) scan of whole body was carried out. The study revealed no metastases. Radiotherapy and irradiation were considered as palliative treatments, but were eventually rejected by oncologists because of local (risk of irradiation of adjacent tissue including coronary arteries) and systemic (risk of unpredictable hypertensive crises) complications of unpredictable severity and unknown benefit. The patient was listed for heart transplantation, which is the only treatment for non-resectable cardiac paragangliomas of such localization.
The clinical presentation of intracardiac paraganglioma can be misleading. Local invasion of the tumor is difficult to rule out. In localization where resection of the tumor or palliative radiation therapy is impossible, urgent heart transplantation should be taken into consideration.







