Purpose
New treatment techniques and early stage diagnoses of endometrial cancer have led to improved cancer-related survival, with increases in treatment-related adverse sequelae, which often negatively affect patients’ quality of life [1,2]. Surgery, consisting of a total hysterectomy and bilateral salpingo-oophorectomy, with or without lymph node dissection, is the mainstay treatment of endometrial carcinoma [3,4]. As reported in a previous meta-analysis [5], adjuvant external-beam radiotherapy (EBRT) is detrimental in low-risk endometrial cancer (stage IA-IB, grade [G] 1-2, FIGO 1988), it does not affect overall survival (OS) in intermediate-risk disease (stage IC G1-2 or stage IB G3, FIGO 1988), and it provides a significant OS benefit in high-risk disease (stage IC G3, FIGO 1988).
Different studies reported that vaginal brachytherapy (VBT) alone can be used as adjuvant treatment in intermediate-risk disease (stage IB G1-2 disease, stage IA G3 disease, and stage IC G1-2 disease), with very good results in terms of local control and toxicity [6,7,8,9].
The adjuvant treatment should be determined by risk factor assessments such as grading, myometrial invasion, lymphatic vascular space invasion, tumor size, lymph node status, tumor extension to the cervix, and age. In uterine confined disease with deep myometrial invasion, stage IB G3 and stage IC G3, the risk of distant metastases is appreciable despite adjuvant radiotherapy (RT). Adding chemotherapy to adjuvant RT in this subset of patients may improve OS by decreasing distant metastases [10]. Adjuvant intravaginal brachytherapy is often preferred due to very good local control and acceptable acute and late toxicities [11,12,13,14,15]. Therefore, VBT is not without acute and long-term side effects and there are different grading systems for the assessment of clinical vaginal toxicity [16,17,18,19]. Frequently, vaginal side effects are a consequence of damage to the vaginal mucosa, the connective tissues and the small blood vessels. One of the most common acute toxicities is vaginal inflammation, which can be manifested by redness, edema, pain, or an increase in vaginal discharge due to damage to the vaginal mucosa [20,21]. The loss of capillaries and impaired microcirculation results in mucosal atrophy, telangiectasias, agglutinations, and easy bleeding. Increased collagen production leads to a loss of elasticity, circumferential fibrosis, and adhesions that can lead to contractions, shortening, narrowing and, in rare cases, complete obliteration of the vagina [22,23,24]. Furthermore, iatrogenic menopause may exacerbate these abnormalities [25,26,27].
Taken together, these adverse vaginal sequelae may increase pain at the time of vaginal examinations, interfere with sexual functioning, be associated with dyspareunia, and negatively affect the patient’s quality of life [28,29]. The aim of this review was to describe acute and late vaginal toxicity (primary endpoint) and local control (secondary endpoint) in patients with low/intermediate-risk endometrial cancer who underwent primary surgery and adjuvant VBT, with or without chemotherapy.
Material and methods
Search strategy
In September 2017, we conducted a comprehensive literature search of the following electronic databases: PubMed, Web of Science, Scopus and Cochrane library. The databases research was made with a combination of following key words: “vaginal brachytherapy”, “toxicity”, and “endometrial cancer” in all fields in databases research. The limit period of research included the articles published from September 1997 to September 2017.
Study selection
In this systematic review, we included randomized trials, non-randomized trials, prospective studies, retrospective studies, and case series of patients affected by endometrial cancer, who underwent surgery treatment and adjuvant vaginal BT. Single case reports and small case series with less than 16 cases were excluded. Moreover, we excluded studies reporting on patients with diagnoses different from endometrial cancer, palliative BT treatment, and interstitial BT treatment. In case of duplicated datasets (e.g., multiple articles from the same study group or institution, related to the same treatment on the same cohort of patient), only the work with the longest follow-up and the greatest number of patients were included.
Data extraction and analysis
Data extraction was performed by one reviewer and checked by a second reviewer. Subsequently, all papers obtained after database research were selected by two reviewers. The first selection was performed by title and abstract reading of each article by each reviewer independently. Then, after the first selection by title and abstract reading, full text of all retrieved papers was reviewed to select suitable articles for the review. After careful selection of articles suitable for the review, we obtained the following information from each report: author identification, year of publication, medical center, study design characteristics, study population, number of patients, age, sex, histological diagnoses, BT technique, total dose, dose for fraction, delivered dose, local control, toxicity, grading scale of toxicity used for each study, and follow-up time. Regarding late and acute toxicities, we considered the study used the following toxicity grading scale: late effects in normal tissues subjective, objective, management and analytic scales (LENT-SOMA) [16], Chassagne [17], late radiation morbidity scoring schema of the Radiation Therapy Oncology Group and the European Organization for Research and Treatment of Cancer (RTOG/EORTC) [18], and NCI common terminology criteria for adverse events (CTCAE) [19].
Statistical analysis
Before data performing, an exploration phase of the data was carried out; the categorical data were described by frequency and percentage, whereas continuous data by mean, median, and range. If necessary, after data exploration, analysis and calculation of frequencies, median and range was performed due to description of end points of the review.
All analyses were performed using the Statistical Package for Social Science (SPSS) version 22 software.
Results
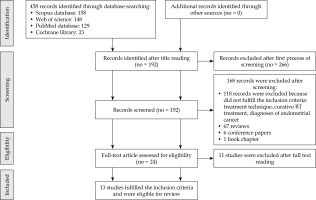
The authors’ searches generated a total of 458 results. Through a process of screening, 13 publications were selected for the review. Of 443 reports excluded for this review, 266 were excluded after the first process of screening. After the second process of screening, 168 reports were excluded; 118 reports were excluded because they did not fulfil the inclusion criteria, 67 consisted of reviews, six consisted of conference papers, and one was a book chapter.
Figure 1 shows the flowchart of the systematic literature search process. After the full text review, 11 records were excluded because they did not fulfil the inclusion criteria: different treatment techniques (such as the use of low-dose-rate brachytherapy, interstitial brachytherapy, and external beam radiation therapy with LINAC), number of patients treated, palliative treatment, and diagnoses of endometrial cancer. Therefore, 13 studies fulfilled the inclusion criteria and were included in the review. The characteristics of these studies are summarized in Table 1.
Table 1
Summary of studies where high-dose-rate endovaginal brachytherapy was used for the treatment of low/intermediate-risk endometrial cancer
| Study(year) | No of pts | FIGOstage | Age(mean, range) | Dose(Gy) | Fractions | Frequency | Size of cylinder(mm) | LC (%) | Follow-up(median) |
|---|---|---|---|---|---|---|---|---|---|
| Laliscia et al. [56] (2016) | 126 | I-III | 67 (27-90) | 21 | 3 | 2-3/week | 25, 35 | 93 | 29 months |
| MacLeod et al. [7] (1998) | 143 | I-III | 62 (38-90) | 34 | 4 | 2/week | 25, 30, 35, 40 | 96 | 83 months |
| Sorbe et al. [8] (2005) | 290 | I | 64.5 (40-89) | 15, 30 | 6 | 2-4/week | 20, 25, 30 | 98.5 | 60 months |
| Sorbe et al. [9] (2009) | 319 | I | 68 (41-88) | 18, 22, 24 | 3, 6 | 2-3/week | 20, 25, 30 | 98.4 | 60 months |
| Chong et al. [10] (2008) | 173 | I | 64 (36-91) | 22 | 4 | 2-3/week | 20, 23, 26, 30 | 96.5 | 38 months |
| Onsrud et al. [14] (2001) | 217 | I-II | 61 (32-85) | 22 | 4 | 5/week | 20, 25, 30, 35, 40 | 99 | 84 months |
| Qian et al. [27] (2017) | 375 | I-II | 65 (43-94) | 14, 18, 21 | 2, 3 | 2-3/week | 20, 23, 26, 30 | 93 | 18 months |
| Greven et al. [34] (2004) | 46 | I-III | – | 18 | 3 | 2-3/week | – | 97 | 29 months |
| Rios et al. [35] (2015) | 154 | I-III | 69 (39-90) | 10, 20 | 2, 4 | 2-3/week | 20, 25, 30, 35 | 97.9 | 47 months |
| De Boer et al. [36] (2015) | 213 | I-II | 69 (46-89) | 21 | 3 | – | – | 97.5 | 84 months |
| Landrum et al. [37] (2014) | 23 | I-II | 69 (46-81) | 21 | 3 | 3/week | – | 87 | 36 months |
| Rovirosa et al. [38] (2012) | 112 | I-III | 66 (39-90) | 10, 20, 30 | 2, 4 | 1-2/week | – | 100 | 30 months |
| Nout et al. [6] (2010) | 213 | I-II | 70 | 21 | 3 | 2-3/week | – | 100 | 45 months |
Doses and fractionation
Compared with EBRT, HDR endovaginal BT has some advantages regarding dose distribution. Brachytherapy is a better conformal therapy that allows an improvement of dose delivery to the target and sharping dose fall-off outside the treatment area [30,31,32,33]. Different studies assessed the dose-effect relationships in vaginal BT [30,31].
In order to evaluate the entire vaginal dose, the Groupe Européen de Curiethérapie and the European Society for Radiotherapy and Oncology (GEC-ESTRO) suggested anatomical reference points as surrogates for the dose distribution along the vaginal axis: one at the mid-vagina (at the level of the posterior-inferior border on the symphysis (PIBS +2 cm), one at the transition from mid to lower vagina (PIBS), and one at the lower region of the vagina (PIBS –2 cm). PIBS points may contribute to finding dose-effect relationships [30].
The Embrace collaborative group reported that dose to 2 cc (D2cc) of the vagina does not correlate with post-radiation vaginal side effects in patients treated with BT for cervical cancer, but an EBRT dose > 45 Gy/25 fractions is a risk factor for vaginal stenosis, and the planning aim of < 65 Gy of 2 Gy equivalent dose (EBRT plus BT dose) to the recto-vaginal reference point is proposed in order to reduce side vaginal effects. In this study, all received EBRT plus BT and the calculation of 2 Gy equivalent dose (EQD2) was performed using the linear-quadratic model with a/b = 10 Gy for tumor and a/b = 3 Gy for late normal tissue damage [30,31].
For these reasons, using BT technique, a high-dose can be given for fractions sparing normal tissue with acceptable acute and late toxicities, as reported through different data in the literature [34,35,36,37,38,39,40,41,42].
Hypofractionated HDR endovaginal BT regimens, which reduce the number of treatment fractions compared to the conventional regimen (conventional regimen consists delivering a dose of 2 Gy for fraction once a day), have been shown to achieve very good local control without increasing side effects.
Different hypofractionated regimens for the treatment of low/intermediate-risk endometrial cancer have been reported in the literature [9,10,21,22,23,24,25,26,27,28,31,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56]. The most commonly described hypofractionated course consists of a total dose of 21 Gy delivered in three fractions two/three times per week, with very good local control and acceptable acute and late toxicities. For the treatment of vaginal cuff after surgery, the proximal (5 cm) of the vagina was treated. The dose prescription point was at the vaginal surface and at 0.5 cm depth [6,10,11,37,40,41,48,56]. Other authors reported their experiences in the treatment of low/intermediate-risk endometrial cancer using adjuvant HDRBT and different fractionation schedules, such as: 22-24 Gy delivered in six fractions two/three times per week, 18 Gy/three fractions two times per week, 30 Gy/six fractions two/four times per week (Sorbe et al. [8,9]), 34 Gy/four fractions two times per week, 22 Gy/four fractions two/three times per week (Chong et al. [10] and Onsrud et al. [14]), and 20-30 Gy/four fractions one/two times per week (Rios et al. [35]), with similar side effects and local control. Table 1 summarizes the studies with total doses and doses for fraction used for the treatment of low/intermediate-risk endometrial cancer.
Local control
Different data in literature reported excellent local control rate in patients with low/intermediate-risk endometrial cancer treated with HDR endovaginal BT. The local control rate is variable reported with a wide range from 87% Landrum et al. [37] to 93-100% [6,15,23,26,27]. The main factors that may influence local control consist of total dose prescription, doses for fractions, overall treatment time, depth of myometrium invasion, histological type, grading, and lymph vascular infiltration [6,7,8,9,10,11,12,13]. Landrum et al. [37] reported a recurrences rate of 13% in 23 patients affected by intermediate-risk endometrial cancer treated with HDR-BT after a median follow-up of 36 months. All patients who experienced relapse had more than one risk factors for endometrial cancer; one patient at histological examination resulted papillary serous carcinoma type and G3, the other patients were stage IIA and G3, third reported myometrium infiltration > 90% and lymphovascular space invasion (LVSI), the last patient with recurrence resulted papillary serous carcinoma type and G3 at histological examination.
The data reported by Qian et al. [27] and Laliscia et al. [56] showed a recurrences rate of 7% after a median follow-up of 18 months and 29 months, respectively. Study of Laliscia et al. [56] included patients with a FIGO stage III (2.4%) unfit to receive EBRT and/or chemotherapy because of comorbidity and age (older than 80 years old); the recurrence rate of patients with FIGO stage > 1C was 18.75%. Finally, the median of local control rate after HDR BT in 10 studies analyzed resulted in 98% (Table 1).
Acute and late vaginal toxicity
There are few data in the literature concerning acute and late toxicities after endovaginal HDR-BT. As reported in different data in the literature, HDR endovaginal BT is very well tolerated, even using different hypofractionated regimens [34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56]. As shown in Table 2, endovaginal acute toxicity varies widely from 8.7% (Qian et al. [27]) to 20.6% (Laliscia et al. [56]). The respective biologically effective doses {BED (a/b = 3)} were 46.7-70 Gy3 and 70 Gy3; calculated EQD2 resulted in 38-42 Gy and 42 Gy, respectively. All acute toxicities were G1-G2 and the most common early side effects due to HDR-BT treatment were vaginal inflammation, vaginal irritation, dryness, discharge, soreness, swelling, and fungal infection [27,56].
Table 2
Summary of acute and late vaginal toxicities after high-dose-rate endovaginal brachytherapy in patients with low/intermediate-risk endometrial cancer
| Author(year) | Acute vaginal toxicity | Late vaginal toxicity | Score used | ||||
|---|---|---|---|---|---|---|---|
| G1-G2 (%) | G3-G4 (%) | Type of toxicities | G1-G2 (%) | G3-G4 (%) | Type of toxicities | ||
| Laliscia et al. [56] (2016) | 20.6 | 0 | Vaginal inflammation Dyspareunia | 23 | 0 | Fibrosis Telangiectasias Dryness Stenosis | CTCAE v. 4.2 |
| MacLeod et al. [7] (1998) | – | – | – | 15.4 | 0 | Discharge | RTOG/EORTC |
| Sorbe et al. [8] (2005) | – | – | – | 24.6 | 0 | Vaginal discharge Dryness Itching Discharge Bleeding | – |
| Sorbe et al. [9] (2009) | – | – | – | 7.5 | < 1 | Slight atrophy Bleedings Fibrosis Stenosis | – |
| Chong et al. [10] (2008) | – | – | – | 12.7 | 0 | Stenosis Bleeding | CTCAE v. 3.0 |
| Onsrud et al. [14] (2001) | – | – | – | 24.4 | 0 | Stenosis | Chassagne |
| Qian et al. [27] (2017) | 15.8 | 0 | Vaginal irritation Dryness Discharge Soreness Swelling Fungal infection | 13.6 | 0 | Stenosis | CTCAE v. 4.0 |
| Greven et al. [34] (2004) | 11.5 | – | – | 26.1 | – | – | RTOG/EORTC |
| Rios et al. [35] (2015) | 8.7 | 0 | – | 27.7 | 0.07 | – | LENT-SOMA |
| De Boer et al. [36] (2015) | – | – | – | 23.4 | 0 | Dryness Short or narrow vagina | EORTC QLQ-C30 |
| Landrum et al. [37] (2014) | – | – | – | 13.1 | – | Dyspareunia Stenosis Dryness | CTCAE v. 3.0 |
| Rovirosa et al. [38] (2012) | 10.9 | 0 | – | 24.9 | 0.09 | Stenosis | RTOG/EORTC |
| Nout et al. [6] (2010) | 25.2 | 0 | – | – | 2 | Atrophy Stenosis Shortening or narrowing | EORTC-RTOG |
[i] RTOG – Radiation Therapy Oncology Group; NCI – National Cancer Institute; CTCAE – common terminology criteria for adverse events; EORTC – European Organization for Research and Treatment of Cancer; LENT – late effects normal tissue task force; SOMA – subjective, objective, management, analytic scale
Regarding late toxicity (Table 2), the current data in the literature report that the treatment is very well tolerated in patients with low/intermediate-risk endometrial cancer. The main side effects consist of grade G1-G2, with a wide range from 7.5% (Sorbe et al. [9]) to 27.7% (Rios et al. [35]). In the Rios et al. [35] study, the HDR VBT was given after EBRT in 63.8% of patients and the biologically effective prescribed doses were 92 Gy3; the calculated EQD2 was 55.4 Gy.
In the Sorbe et al. [9] study, the BED was wide from 15.1 to 56 Gy3 and the calculated EQD2 resulted in < 33.6 Gy. The most common late G1-G2 side effects reported consisted of vaginal discharge, dryness, itching, bleeding, fibrosis, telangiectasias, stenosis, short or narrow vagina, and dyspareunia.
G3-G4 late vaginal toxicities have been reported only in a few cases (four studies) and resulted in less than 2% of cases (Nout et al. PORTEC-2 study [6]), consisting of slight atrophy, bleeding, and stenosis [6]. Table 2 describes the summary of acute and late vaginal toxicity after endovaginal HDR brachytherapy.
Discussion
Laparotomy, peritoneal washing, total extra-fascial hysterectomy, and bilateral salpingo-oophorectomy with or without pelvic ± aortic lymphadenectomy is the standard treatment for endometrial cancer [2,3,4]. Adjuvant RT, with or without chemotherapy, is often indicated in patients after complete surgical staging. Risk factors such as grading, myometrial invasion, lymphatic vascular space invasion, tumor size, lymph node status, tumor extension to the cervix, and age should be considered for adjuvant treatment of endometrial cancer. In patients with uterine confined disease and deeply myometrial invasion, stage IB G3 and stage IC G3, adding chemotherapy to adjuvant RT may improve the OS [5,6,7,8,9,10,11,12,13,14,15,57,58,59,60].
The Postoperative Radiation Therapy in Endometrial Cancer (PORTEC 2) [6] trial randomly allocated 427 patients with intermediate/high-risk endometrial cancer to receive either adjuvant vaginal BT (21 Gy with high-dose-rate [HDR] or 30 Gy with low-dose-rate [LDR]), or adjuvant pelvic EBRT (46 Gy) [8,9]. The two arms had similar 3-year vaginal recurrence rates (0.9% for vaginal BT vs. 2% for EBRT) and 3-year OS rates (90.4% vs. 90.8%), whereas patients enrolled into vaginal BT arm experienced better social functioning, less bowel toxicity, and better quality of life (QOL). In vaginal BT arm, G3-G4 late vaginal toxicities resulted in less than 2% and consisted of slight atrophy, bleedings, and stenosis.
HDR VBT alone is often preferred as adjuvant therapy in patients with intermediate-risk endometrial cancer due to excellent local control and low-rate of acute and late toxicities [6,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,60]. However, there is a lack of data in literature concerning acute and late toxicities after HDR vaginal BT as exclusive post-operative radiotherapy.
Different data in literature showed one correlation between total dose prescribed, doses for fractions, length of vagina treated, and vaginal late toxicity [8,48,49,50,51,52,53,54,55,56,60,61,62,63,64,65]. Sorbe et al. [8] in 2005 reported results of a randomized study using two different dose-per-fraction in patients with IA-IB stage endometrial cancer treated with endovaginal HDR brachytherapy. Overall, 290 patients were treated vaginal applicators with diameters of 20-30 mm. The dose per fraction prescribed was randomly set to 2.5 Gy (total dose of 15.0 Gy) or 5.0 Gy (total dose of 30.0 Gy). One hundred forty-four patients were treated with 2.5-Gy fraction and 146 5.0-Gy fractions. The dose prescription was at 5 mm depth from the surface of the vaginal cylinder using the HDR technique. The overall locoregional recurrence rate was 1.4% and the rate of vaginal recurrences 0.7%. There was no difference between the two randomized groups. The vaginal shortening, mucosal atrophy, and bleedings were highly significant (p < 0.000001) in the 5.0-Gy group after 5 years.
In Park et al. study [43], predictors of vaginal stenosis after intravaginal HDR brachytherapy in endometrial carcinoma were analyzed. After a median follow-up of 12.9 months, all 101 patients analyzed were disease-free. Highest vaginal stenosis (VS) grades were zero in 67%, one in 26%, two in 6%, and three in 1%. Multivariable analysis revealed that proportion of vagina treated > 60% (odds ratio [OR]: 3.48; p = 0.009) and total dose > 14 Gy (OR: 4.27; p = 0.015) were independent predictors of grade ≥ 1 vaginal stenosis (VS), and lack of consistent dilator use was an independent predictor of grade ≥ 2 VS (OR: 5.60; p = 0.047). Authors concluded that higher total dose to a larger proportion of the vagina were more likely to develop grade ≥ 1 vaginal stenosis.
MacLeod et al. [7] used HDR vaginal BT only (34 Gy in four fractions prescribed to vaginal mucosa in a two-week period) in 143 patients. Five-year disease-free survival (DFS) and five-year OS were 100% and 88% for stage IA (FIGO 1988), 98% and 94% for stage IB, 100% and 86% for stage IC, and 92% and 92% for stage IIA, respectively. The overall vaginal recurrence rate was 1.4%, and the overall late-toxicity rate was low, with no grade ≥ 3 toxicities. The vaginal toxicity (grade 1 and 2) was reported in 15.4% of patients.
Alektiar et al. [12] retrospectively assessed 382 patients with stage IB-IIB endometrial cancer treated with surgery followed by HDR vaginal BT (21 Gy, given in three fractions at a two-week interval, and the dose was prescribed to a depth of 0.5 cm from the vaginal surface). The five-year vaginal/pelvic control rate was 95% and on multivariate analysis, loco-regional failure rate correlated with age ≥ 60 years old and lymph-vascular space involvement invasion. Grade 3 or higher late toxicities were observed in three cases (0.8%) and consisted of vaginal necrosis, chronic cystitis, and urethral stricture, respectively.
Different authors reported that the local treatment appears to reduce vaginal atrophy and related symptoms in women treated with radiotherapy for gynecological cancers. This local therapy may favor regeneration and epithelial proliferation, and improve vaginal trophism, elasticity, and adequate lubrication. Finally, the consistent dilator use may also be protective against grade ≥ 2 of vaginal stenosis [48,54,55,56,60].
The limitations of the present review are due to the lack of data in the literature regarding the study of late and acute vaginal toxicities after adjuvant intravaginal HDR BT. Many of the studies analyzed were retrospective, and acute and late toxicity was not described in detail. Further, the studies were performed in different countries using different grading scale, dose prescription, and doses per fraction.
Conclusions
There is a lack of data in the literature regarding the incidence and preventative strategies of vaginal toxicity after adjuvant HDR endovaginal BT. The authors’ data suggest that HDR adjuvant endovaginal BT (with or without chemotherapy) is very well tolerated with very good local control disease in low/intermediate-risk endometrial cancer. Patients with high-risk disease (stage IC, G3, or clear cell histology) should be considered for more extensive treatment. Further prospective studies with a higher number of patients and longer follow-up to evaluate acute and late toxicities after endovaginal HDR BT are recommended.