Purpose
Prostate ductal adenocarcinoma (PDA) represents a rare variant of prostate cancer in patients with non-metastatic as well as metastatic disease, and regardless of the stage, PDA behaves more aggressively and has a high mortality rate [1, 2]. The disease has been associated with a higher likelihood of extra-prostatic extension compared with acinar adenocarcinoma, positive surgical margins, and shorter time to recurrence after radical prostatectomy [2-4].
At this moment, an optimal treatment for PDA remains unknown [5]. In particular, the role of low-dose-rate brachytherapy (LDR-BT) in the treatment of PDA has not been described previously. We have shown good clinical outcomes by escalating radiation dose in high-risk and very high-risk prostate cancer patients, including five pelvic lymph node metastasis cases [6]. These patients achieved a biochemical failure-free survival (BFFS) rate of 95.2% at five years by a tri-modality therapy with biologically effective dose (BED) ≥ 220 Gy: a combination of LDR-BT, external beam radiotherapy (EBRT), and short-term androgen deprivation therapy (ADT) [6]. Furthermore, this author has also shown that T4 prostate cancer with pelvic lymph node metastasis (T4N1 disease) can be cured in the same way by using seminal vesicle seed implantation [7]. Until now, there has been no definitive report of a complete cure of bulky, locally advanced very high-risk PDA.
This case report highlighted the effectiveness of a BED ≥ 220 Gy of high-dose radiotherapy, using LDR-BT in combination with external beam radiotherapy (EBRT), with short-term ADT (six months of neoadjuvant and adjuvant ADT before and after LDR) in very high-risk locally advanced bulky PDAs through a long-term follow-up.
Case reports
Case 1
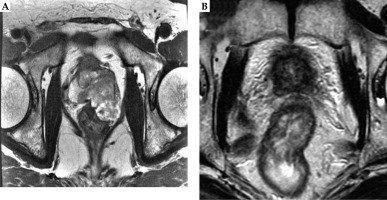
A 64-year-old man with T3b PDA was classified as very high-risk prostate cancer (Table 1). The patient was referred to this author’s specialized outpatient clinic for prostate brachytherapy in 2009 because of his elevated PSA level (16.6 ng/ml). During the first interview, the patient complained of difficult urination. A digital rectal examination showed a hard nodule extended over the rectal wall on the left side of his prostate. Magnetic resonance imaging (MRI) revealed a bulky prostate tumor extending to the rectum (Figure 1A). Prostate biopsy was performed by his care team: seven trans-rectal biopsy specimens all showed PDA with a Gleason score of 4+4. After neo-adjuvant ADT for six months, the patient received LDR-BT with seminal vesicles implantation. Seminal vesicle implantation method has been described previously in detail [7]. Briefly, LDR seed implantation was conducted with 125I seeds using real-time ultrasound guided technique, as described later. Sixty seeds (OncoSeed; Nihon Medi-Physics Co. Ltd., Tokyo, Japan), including six seeds for seminal vesicles, were implanted. Seed activity used during implantation was 0.284 mCi. Dosimetric parameters at one month after LDR-BT, dose of supplemental EBRT, and total BED in the two cases are shown in Table 2. In case 1, the patient received supplemental EBRT, with 39.6 Gy to the entire prostate and seminal vesicle (1.8 Gy × 22 fractions). The total BED (α/β ratio of 2 Gy) in case 1 was 238.3 Gy.
Table 1
Patients’ characteristics
Table 2
Dosimetric parameters at one month after low-dose-rate brachytherapy (LDR-BT), dose of supplemental external beam radiotherapy (EBRT) and total biologically effective dose (BED)
| Parameter | Case 1 | Case 2 |
|---|---|---|
| Prostate D90 (Gy) | 154.7 | 129.2 |
| V100 (%) | 99.6 | 97.9 |
| UD30 (Gy) | 217.0 | 154.3 |
| R100 (cc) | 0.52 | 0.03 |
| Dose of EBRT (Gy) | 39.6 | 45.0 |
| Total BED (Gy) | 238.30 | 220.60 |
[i] D90 – minimal dose (Gy) received by 90% of the prostate; BED – biologically effective dose; V100 – the percentage of the prostate volume receiving 100% of the prescribed minimal peripheral dose; UD30 – minimal dose (Gy) received by 30% of the urethra; R100 – rectal volume (ml) receiving 100% of the prescribed dose
Case 2
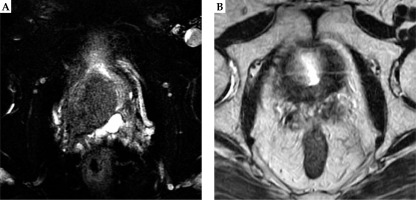
A 70-year-old man with T3b PDA was classified as very high-risk prostate cancer (Table 1). The patient originally consulted a regional urologist for his elevated PSA of 5 ng/ml in 2011. The urologist did not conduct any further examination, such as rectal digital examination, trans-rectal ultrasound, or MRI. Six months later, the patient complained of difficulty in urination and consulted the same urologist. PSA level of the patient was now elevated up to 6 ng/ml. However, no further examination was conducted. In 2013, PSA of the patient was elevated up to 11 ng/ml. Finally, the patient was diagnosed with a Gleason 4+4, T3b clinical stage prostate cancer by trans-rectal biopsy in a regional hospital. The patient sought this author’s medical advice and treatment. Then, he visited our specialized outpatient clinic for prostate brachytherapy in 2013. On the first interview, a digital rectal examination showed a hard nodule that extended over the entire prostate. Eleven trans-rectal biopsy specimens obtained from the regional hospital all diagnosed PDA with a Gleason score of 4+4. MRI showed bulky locally advanced prostate cancer (clinical T3b) (Figure 2A). After neo-adjuvant ADT for six months, the patient received LDR-BT with seminal vesicles implantation. LDR-BT was conducted in the same way as it was conducted in case 1. A total of 74 seeds (OncoSeed; Nihon Medi-Physics Co. Ltd., Tokyo, Japan), including 15 seeds for seminal vesicles were implanted. Activity of the implanted seeds was 0.284 mCi. Dosimetric parameters at one month after LDR-BT, dose of supplemental EBRT, and total BED of the two cases are shown in Table 2. In case 2, the patient received supplemental EBRT, with 45 Gy to the whole pelvis (WP) (1.8 Gy × 25 fractions). The total BED (α/β ratio of 2 Gy) in case 2 was 220.6 Gy.
Low-dose-rate brachytherapy protocol
The details of LDR-BT protocol have been described previously by the author [7]. Radioactive seeds were implanted into the prostate using a Mick applicator (Mick Radio-Nuclear Instruments, Inc., Mount Vernon, NY, USA). Seeds were implanted successively, and dosimetry plan was updated after each seed implant before continuing with the next one, so that the plan evolved dynamically as seeds were implanted. Planning was performed with VariSeed v.8.0 planning system (Varian Medical Systems, CA, USA). Post-implant dosimetry with computed tomography (CT) and MRI guidance was carried out at one month after seed implantation. BED values for treatments, including both implants and EBRT were calculated by adding BED calculated for each treatment [8]. Further information regarding BED calculations and the rationale for selecting an α/β ratio of 2 Gy for prostate cancer have been previously described in detail [9].
Outcomes of both the cases
Both patients received adjuvant ADT for six months after LDR-BT (Table 1). ADT consisted of gonadotropin-releasing hormone agonist injections and antiandrogen. The outcomes were remarkably similar for the patients. Both patients’ PSA levels temporarily increased after cessation of ADT, followed later by continual reductions in PSA levels. At their latest follow-up evaluations (case 1 at 11.5 years after completion of EBRT, and case 2 at 8 years after completion of EBRT), PSA values in both the patients were 0.01 ng/ml (Table 3). Serum testosterone values of both the patients were within normal range (Table 3), indicating that the effect of ADT on the above-mentioned low PSA level was negligible. MRI at their latest follow-up showed shrinkage of their bulky tumors (Figures 1B and 2B).
Table 3
Clinical outcomes of the two investigated cases
Both the patients recovered and remain healthy without biochemical or clinical failure as well as without bowel or urinary problems at 11.5 years and 8 years after the treatment in case 1 and case 2, respectively. For individual data usage, including images and medical history, the author has obtained written informed consent from the patients.
Discussion
Due to the rarity of PDA, the literature on treatment outcomes for PDA is very limited. The level of evidence to guide management for PDA treatment is of low quality. The situation is very similar to that of T4 prostate cancer [7].
Most of the literature is based on demographic data, such as the SEER database study [1]. These reports, including a review article on PDA, consistently reported poor prostate cancer-specific survival in PDA patients [1, 10].
The shared conclusion that can be drawn from these data is that PDA remains a challenging disease to diagnose and treat, and that PDA is associated with a poor prognosis [10, 11]. 1) Patients with PDA, including metastatic disease, tend to present with lower PSA levels than those with prostatic acinar carcinoma; 2) Following definitive therapy of localized PDAs with radical prostatectomy or radiotherapy, the majority of PDA cases return with local recurrence and distant metastasis [10, 11].
Concerning the issue (1), in case 2 of this case report, the diagnosis of prostate cancer was delayed for two years because of the lack of a detailed medical examination. This was probably due to the relatively low PSA of the patient with a bulky PDA. The readers of this paper should recognize this finding as a typical diagnostic pitfall in PDA. Concerning the issue (2), even in this era, there is currently no optimal treatment guidelines for PDA, and its’ management recommendations are based on a small number of PDA case series studies [5, 10, 12].
Furthermore, none of the published studies has indicated a successful treatment of very high-risk locally advanced PDAs with a long-term follow-up, such as that demonstrated in this case series. We have previously shown good clinical outcomes by dose escalation in high-risk and very high-risk prostate cancer that included five pelvic lymph node metastasis cases; BFFS rate of 95.2% at 5 years by LDR in combination with EBRT at BED ≥ 220 Gy [6]. The present case report series included a detailed report on pure PDA with extensive local invasion through a long-term follow-up of our previous report on high-risk prostate cancer [6]. Among this author’s 1,238 cases of brachytherapy, only these two cases (0.16%) could be considered as pure PDA. This incidence is similar to that in the SEER database [1]. Molecular pathogenesis of PDA remains largely unknown. Recently, Schweizer et al. reported a high prevalence of DNA repair genes, such as BRCA2, ATM, and MSH2 [13]. Further genetic analysis may lead to new therapeutic strategies.
In case 1, we set the clinical target volume for EBRT to the entire prostate and seminal vesicle, because this case is our early experience with cases of this type. The current definitive policy of the author for the treatment of T3b or T4 prostate cancers is LDR combined with WP-EBRT.
Therefore, combining the experience described in our previous reports on high-risk prostate cancer with or without pelvic lymph node metastasis [6], including prostate cancer with nodular bladder invasion (T4N1 disease) [7], with that described in the present paper, we conclude that LDR in combination with EBRT at a BED ≥ 220 Gy is an optimal treatment for very high-risk locally advanced PDA. The toxicity encountered with this method is minimal if the treatment is conducted properly.