Introduction
In the foetal circulation, mature immune cells are accompanied by relatively abundant immature cell subsets. In case of neonatal pathology, these cells support host resistance and tissue repair additionally providing a prognostic tool [1]. On the other hand, umbilical cord blood (UCB) containing low differentiated, pro-regenerative cells can be applied for allogenic transplantation and tissue repair in various pathologies, e.g. haematological and autoimmune diseases. Advantages of their applications involve better HLA mismatch toleration and lower risk of graft-versus-host disease as compared to adult cells [2]. UCB is prima-rily considered a source of hematopoietic stem cells (HSC), which reside in heterogenic CD34+ cell fraction. The CD34 antigen, cell surface sialomucin is uniquely expressed on the hematopoietic and progenitor stem cells (HSPC) but also on non-hematopoietic cells and is an accepted marker of stemness and immaturity. It plays a role in cell adhesion, rolling, migration, proliferation, differentiation and apoptosis [3, 4]. The numbers of UCB CD34+ cells are critically important for transplantation success of HSCs providing proper conditions to proliferation, mobility, bone marrow homing and engraftment [5, 6]. While subjected to specific expansion techniques, UCB CD34+ cell population can provide large amounts of functional innate immune cells, i.e. neutrophils [7]. Regarding the CD34+ cells, concomitant assessment of the expression of another immaturity marker in humans, the CD133 molecule (prominin-1), allows to distinguish two functionally distinct cell subpopulations important for further applications: CD34+CD133– and CD34+CD133+. CD133+ cells, specifically upregulated in hypoxia and mitochondrial dysfunction, are of significance for immature and tumour cells, cell regeneration and metabolism [8]. Within CD34+ cells population, exhibiting CD133, granulocyte-macrophage progenitors (GMP) were exclusively found [6]. Therefore, quality of this specific cell population reflects the HSPC activity of UCB. It has been demonstrated that obstetric and maternal factors could determine CD34+ and CD34+CD133+ cell counts [6, 9]. Potential effects of obstetric anaesthesia have not been explored.
Broad use of regional methods of anaesthesia and analgesia in obstetrics evoked interest in foetal and neonatal implications of these procedures [10]. Agents applied in these techniques, local anaesthetics (LAs), temporally block neuronal ion channels and nociceptive stimulation. However, they may also exert cytotoxic and pro-apoptotic effects in various cell populations. Depending on the clinical or experimental conditions, these actions were proved to be beneficial (e.g., tumour) or detrimental (e.g., neural injury) [11, 12]. LAs instilled locally or neuraxially penetrate the vessels and in pregnant women via placenta reach foetal circulation influencing viability and function of systemic immune cells. We have shown that LAs are capable of inhibiting reactive oxygen species (ROS) generation in neonatal neutrophils even at low concentrations present in foetal blood during mothers’ epidural blockade. However, the effect is less pronounced and lasts shorter than in adult cells [10].
In immature cells, it was demonstrated that nitric oxide (NO), a pleiotropic signaling and effector molecule, is involved in cell survival and renewal in a concentration dependent manner [13]. LAs are able to influence NO signaling in immune cells, decreasing or increasing NO endogenous generation and expression of NO synthase (NOS) isoforms depending on the cell type [14, 15]. Whether this process occurs in not finally differentiated cells and correlates with the cell life span remains unclear.
This study aimed at assessing the LAs influence on the viability and intracellular NO production of UCB CD34+CD133– and CD34+CD133+ cell populations.
Material and methods
The study was performed according to the Bioethical standards of Helsinki Declaration and its latest revision. After obtaining local Bioethical Commission approval (780/13, 659/15, Poznan University of Medical Sciences) and mothers’ written informed consent, the cord blood samples (18 ml) from healthy, full term neonates (n = 19) were drawn immediately after cord clamping and proceeded within 1 h. Mothers were non-smoking, healthy individuals neither receiving any medication nor requesting neuraxial blockade for labour pain control.
Mononuclear cell isolation
Mononuclear cells (MNC) were isolated form UCB by density gradient centrifugation using Gradisol L (d = 1.077 g/cm3; Aqua-Med, Poland) and centrifuged at 400 g, RT, for 30 min. The buffy coat was collected, PBS-washed, centrifuged and resuspended in 2 ml Hank’s balanced salt solution without Ca2+/Mg2+ (HBSS; Institute of Immunology and Experimental Therapy, Polish Academy of Sciences, Wroclaw, Poland), and then counted using Bürker chamber. MNC purity and viability on Trypan blue dye exclusion were > 95% and > 98%, respectively. Samples of 3 × 106 cells were used for further analysis. No erythrocyte lysis was necessary.
Incubation with local anaesthetics
Stock solutions of 5 mM bupivacaine (Bupivacaine hydrochloride monohydrate; Sigma-Aldrich, MO, USA), 16 mM lidocaine (Lidocaine hydrochloride monohydrate; Sigma-Aldrich), and 7 mM ropivacaine (Ropivacaine hydrochloride monohydrate; Sigma-Aldrich) prepared from water dissolved agents were sterilized and refrigerated until use. Before experiments, LAs were diluted in HBSS to obtain final equipotent concentrations: 0.0005, 0.005 and 1 mM bupivacaine; 0.002, 0.02, and 4 mM lidocaine; and 0.0007, 0.007 and 1.4 mM ropivacaine. The lowest concentrations of bupivacaine and ropivacaine reflected those observed in foetal circulation during epidural labour analgesia [16]. MNC were incubated with LAs under standard conditions (37oC; 5% CO2; HeraCell 150; ThermoScientific, MA, USA) for 4 h.
Flow cytometry
After incubation with LAs, MNC were centrifuged and incubated with monoclonal antibodies conjugated with fluorochromes: anti-CD34– PE (clone AC136, Miltenyi Biotec, Bergisch Gladbach, Germany) and anti-CD133-APC (clone 293C3, Miltenyi Biotec, Bergisch Gladbach, Germany) in the dark, 4oC, for 10 min. Cells were washed twice with cold PBS supplemented with 2 mM EDTA and 0.5% FCS. Progenitor cells were identified by flow cytometry (FACS CantoII; BD Biosciences, San Jose, CA, USA) based on forward (FSC) vs. side scatter (SSC) characteristics and the presence of surface antigens.
Cell viability
Apoptotic cells were assessed using annexinV-FITC/ 7-AAD assay (BD Pharmingen, USA). After antibody staining, cells were washed twice with cold PBS, resuspended in Binding Buffer, incubated with Annexin V-FITC (5 µl) and 7-AAD (5 µl) in the dark, RT for 15 min, and then analysed by flow cytometry within 1 h.
In both studied cell populations, viable (Annexin V–/ 7AAD–), early apoptotic (Annexin V+/7AAD–), late apo-ptotic (Annexin V+/7AAD+), and necrotic cells (Annexin V–/7-AAD+) were distinguished. Compensation was set up and calculated using FACS Diva Software (BD Biosciences).
Intracellular NO production
Mononuclear cells were incubated with 2.5 µM DAF-2DA (4,5-Diaminofuorescein diacetate solution; Sigma-Aldrich) in dark, RT, for 2 h. Fluorescence from triazolofluorescein (DAF-2T) reflected intracellular NO generation. DAF-2T positive events were gated as the CD34+CD133+ and CD34+CD133– cell populations with high fluorescence in FL1/DAF-2T FITC channel. Gates of high fluorescence were set in comparison to non-fluorescent control.
Samples of 2 × 106 MNC were collected. The number of analysed cells was the total number of cells per 2 × 106 MNC.
Statistics
Statistical analysis was performed using Statistica version 12 (StatSoft, Tulsa, OK, USA), JumpPro (jmp-11.0) 32 bit 11.0.0. (SAS Institute), and GraphPad Prism 6.0 (GraphPad Software, Inc., San Diego, CA, USA).
Multifactorial analysis of variance (ANOVA) was applied for the assessment of LAs impact on cell viability and NO production. Percentage data of cell viability were arcsine transformed to obtain normal distribution. Effects of independent factors: 1) ‘cell population’ – CD34+CD133– and CD34+CD133+ cells, 2) ‘anaesthetic’ – bupivacaine, lidocaine, ropivacaine, and 3) ‘concentration-category’ – concentrations of LAs categorized by their potency: the lowest, middle, the highest, were evaluated. Additionally, interactions between the factors were assessed.
Results
Obstetric and newborn data are presented in Table 1.
Table 1
Obstetric characteristics and the newborn data
Cytometric cell phenotype identification and viability assessment are shown in Figure 1A-F.
Fig. 1
Cytometric identification, viability, and intracellular nitric oxide (NO) generation in umbilical cord blood (UCB) CD34+CD133– and CD34+CD133+ cell populations (n = 19). A) Representative dot plot of UCB mononuclear cells (MNCs) identified by forward and side scatter characteristics (FSC and SSC, respectively). 2 × 106 MNC samples were evaluated. B) Progenitor cells were separated from MNCs using CD34 gate. C) CD133+ and CD133– subsets were identified. Viable (Annexin V−/7-AAD−), early apoptotic (Annexin V+/7-AAD−), late apoptotic (Annexin V+/7-AAD+) and necrotic (Annexin V−/7-AAD+) cells were distinguished in (D) CD34+CD133– and (E) CD34+CD133+ cell populations. F) Percentages of viable (white bars), early apoptotic (light grey bars), late apoptotic (dark grey bars) and necrotic (black bars) CD34+CD133– and CD34+CD133+ cells. More details of multifactorial ANOVA analysis have been summarized in Table 2. G) Intracellular NO production in CD34+CD133– and CD34+CD133+ cells was assessed by flow cytometry and is expressed as mean fluorescence intensity (MFI) of triazolofluorescein (DAF-2T). F, G) Data are the mean ±SD
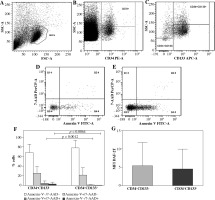
Viability of UCB CD34+CD133– and CD34+CD133+ cells
UCB CD34+CD133– cell population showed higher percentage of late apoptotic and necrotic cells as compared to CD34+CD133+ cells (p = 0.0012 and 0.0064, respectively, multifactorial ANOVA; Fig. 1F, Table 2). Higher percentage of viable CD34+CD133+ cells failed to reach statistical significance (p = 0.0652).
Table 2
Percentage of viable (Annexin V+/7-AAD-), early apoptotic (Annexin V+/7-AAD-), late apoptotic (Annexin V+/7-AAD+), and necrotic (Annexin V+/7-AAD+) UCB CD34+CD133- and CD34+CD133+ cells exposed to bupivacaine, lidocaine and ropivacaine
Nitric oxide generation in UCB CD34+CD133– and CD CD34+133+ cells
Nitric oxide production revealed in CD34+CD133– and CD34+CD133+ UCB cell populations was similar (p > 0.05; Fig. 1G).
Effects of local anaesthetics on cell viability and nitric oxide generation
Local anesthetics did not affect the viability of UCB cells of both phenotypes studied. The effect was comparable between bupivacaine, lidocaine, and ropivacaine. The agents did not change the percentage of viable, early apoptotic, late apoptotic and necrotic cells (Fig. 2A).
Fig. 2
Effects of local anaesthetics (LAs) on viability and intracellular nitric oxide (NO) generation in UCB CD34+CD133– and CD34+CD133+ cell populations (n = 19). A) Percentages of viable (Annexin V−/7-AAD−; white bars), early apoptotic (Annexin V+/7-AAD−, light gray bars), late apoptotic (Annexin V+/7-AAD+; dark gray bars) and necrotic (Annexin V−/7-AAD+; black bars) cells evaluated in CD34+CD133– and CD34+CD133+ populations incubated with bupivacaine, lidocaine and ropivacaine for 4 h. Graphical representation of significant interaction determined by multifactorial ANOVA between the factors of ‘population’ and ‘viability’; all applied concentrations of particular LA were collectively assessed. In CD34+CD133+ cell subset, percentage of live cells was higher than that of CD34+CD133– cells, and percentages of necrotic and apoptotic cells were consequently lower under the exposure of each LA tested. Data are the mean ±SD. Further details are shown in Table 2. B) Intracellular NO production in CD34+CD133– and CD34+CD133+ cells exposed to LAs was assessed by flow cytometry and is expressed as mean fluorescence intensity (MFI) of triazolofluorescein (DAF-2T). Data are the mean ±SD, p > 0.05
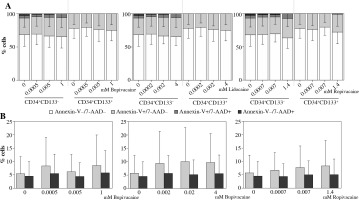
Viability of UCB CD34+CD133– and CD34+CD133+ cells exposed to particular LAs studied were comparatively presented in Table 2 reflecting significant interaction between the factors ‘population’ and ‘viability’. All applied concentrations of particular LA were collectively analysed. Regarding UCB CD34+CD133+ cell population, percentage of live cells was higher than that of CD34+CD133– cells, and percentages of necrotic and apoptotic (early and late) cells were consequently lower under the exposure to each anaesthetic tested.
Intracellular NO production was unaffected under the exposure to all LAs tested in both cell populations studied. No differences between UCB CD34+CD133– and CD34+CD133+ cells in this aspect have been found (Fig. 2B).
Discussion
In the present study, we investigated effects of commonly used aminoamide-type LAs, bupivacaine, lidocaine and ropivacaine, on the cell viability and intracellular NO generation among immature UCB cell populations CD34+CD133– and CD34+CD133+. We observed significant differences in late apoptosis and necrosis between these cell fractions, however, we failed to detect any significant influence of the agents on the phenomena studied.
Viability of CB CD34+ cells varied substantially (over 80% vs. 40%), depending on blood samples delivery and mode of cell isolation. Results obtained in this study corresponded with previously reported values [17]. We observed that in the CD34+CD133+ cells, late apoptosis and necrosis were significantly less frequent than in CD34+CD133– cell population, indicating higher survival potential of the former population. Percentages of early apoptosis corresponding with functional deficits, like decreased clonogenic and transmigration capacity were, however, comparable [18]. Our results indicate that CD34+CD133+ phenotype present with less pronounced propensity to cell death and are consistent with the resistance to apoptosis of CD133+ phenotype [8, 19]. In human UCB MNC subset, CD133+ phenotype showed better radioresistance compared with CD133– cells with the low radiation induced apoptosis and distinct repair signalling [20]. Relative insensitivity to apoptosis of UCB progenitors was reported to depend on active upregulation of caspase 8 and NF-κB-related pathway [19], which in our study analysing UCB cells exposed to inflammatory conditions of natural labour might have been of importance.
In the present study, no difference in intracellular NO generation between both cell populations studied could be observed. NO generation is important for CD34+ cell bio-logy. In adult peripheral blood CD34+ fraction of MNC, NO was produced twice as much as in CD34– cells [21]. Association between NO generation and cell death is also a complex issue. Pro- or anti-apoptotic actions of NO in not finally differentiated cells were demonstrated to depend on the mediator amount and/or its source, endogenous or extracellular [13]. In bone marrow CD34+ cells, Fas-mediated apoptosis was connected with the activity of inducible NOS2 isoform providing higher amounts of NO than the other two constitutive NOS isoforms [22]. In our study, specific inflammatory and hormonal milieu of natural labour and exposure of the studied cell populations to a number of stimuli including cytokines and hormones present in UCB could also influence NO intracellular generation, i.e., upregulating the NOS2 [23]. Human CD34+ stem cells exposed to extracellular NO produced by M1-macrophages which demonstrated prevalent NOS2 activity, presented increased apoptosis and impaired self-renewal [24]. On the other hand, in UCB hematopoietic cells, extracellular NO may also inhibit apoptosis and upregulate a number of transcription factors connected with CD34 [25, 26]. NO is also able to activate cell signaling pathways important for ex vivo expansion of CB HSC [27, 28]. In view of the complexity of NO influence on UCB CD34+ cell survival, a deeper insight identifying expression and activity of specific NOS isoforms should be warranted.
In this study, no significant influence of LAs, bupivacaine, lidocaine and ropivacaine on UCB CD34+CD133– and CD34+CD133+ cell viability was detected. These findings, especially important from a clinical point of view, suggest that in perinatal settings neuraxial blockades should not negatively influence UCB cells important for the neonate and/or for banking purposes.
LAs were repeatedly shown to be able to induce cell death in various cell populations. In immune cells, these agents may exert either cytotoxic or stimulatory effects [29]. As shown in Jurkat cells, association between LAs cytotoxicity and agents’ characteristics, i.e., lipophilicity and clinical potency, was not so evident as in neuronal cells. Structural features like ester or amide linkage and stereospecificity were also of no significance [30]. In clinical conditions, transient apoptotic effect of epidural anaesthesia using lidocaine and ropivacaine in peripheral blood monocytes and lymphocytes was observed [31]. In immature cells, like preadipocytes and mesenchymal stem cells (MSC), cytotoxicity was well documented [32-35]. Commonly used aminoamide-type LAs decreased viability of MSC in agent-, concentration- and time-dependent manner. In this study, underlying reasons for lack of LAs effects on the studied UCB CD34+ cell subpopulations viability might involve: 1) specific experimental conditions applied, agents’ concentrations and time of exposure which were subjected to clinical conditions, 2) distinct UCB CD34+ cell biology, and 3) cell exposure to specific inflammatory and hormonal milieu of natural labour. In murine MSC, ropivacaine at 100 and 250 µM activated early apoptosis, with no detectable effect on late apoptosis only after 24-h exposure [33]. In human MSC, apoptosis was observed as early as after 40 min incubation, however, agents were applied at high concentrations of molar range [34]. Delayed effects after 96 h since 1 h exposure were reported also for molar concentrations [35]. Concentrations applied in this study were much lower, the lowest concentrations reflected real amounts of agent in UCB following maternal epidural blockade, up to the highest ones, millimolar assumed as reference values. Exposure time of 4 also referred to clinical conditions [36]. Reported mechanisms responsible for detrimental effects on MSC viability differed between the agents applied and involved mitochondrial dysfunction typical for lidocaine (also demonstrated in human neutrophils) and depletion of intracellular calcium stores observed under bupivacaine exposure [33, 34, 37]. On the other hand, LAs are able to increase or inhibit ROS generation and to increase intracellular pH (pHi) by blocking voltage-gated proton (VDPC) channels, whereas in CD34+ cells it was demonstrated that early apoptosis is associated with ROS stimulation and pHi decrease [38, 39]. Extracellular acidosis, typical for UCB milieu and also documented in this study, had no effect on the viability of human MSC in the presence of LAs [35]. It is worth mentioning that cytotoxic effects of LAs in human MSC was demonstrated only in monolayer cultures and not in the whole tissue samples, indicating that microenvironment of the experiment may be critical for the results obtained [35]. As CD34+ phenotype prevents adhesion, this feature might possibly contribute to a lack of cytotoxic effects observed in this study [3, 35]. Eventually, natural labour and delivery is connected with delayed neutrophil apoptosis in maternal blood and in UCB cells [40]; this aspect of apoptosis resistance in UCB immature cells should also be considered. Despite no effect on LAs on cell viability, using multifactorial ANOVA, we could observe consistent differences between two studied cell populations concerning viability parameters. This observation supports earlier described distinct reactivity of CD34+CD133– and CD34+CD133+ cell populations with propensity to better survival of CD133+ phenotype.
Originally, we suspected that NO signalling could possibly interact with the viability of UCB CD34+ cells exposed to LAs. In contrast to our previous findings showing upregulation of intracellular NO generation in UCB neutrophils by LAs, we could not observe here any alteration in NO production under the influence of all LAs tested. This preliminary observation warrants further analysis elucidating specific cellular sources of NO generation.
We are aware of some limitations. Firstly, we applied limited phenotyping of the studied cell populations, not allowing to discriminate between different progenitor subsets. However, for the purpose of this study more detailed assessment was not essential, as we wanted to answer a general question of the influence of LAs on the viability of CD34+ cells. In the literature, exploration of different obstetric factors on the HSPC content in CB was also addressed [6]. Secondly, we applied limited concentration of LAs in order to address typical clinical situation. Thirdly, the study was performed using UCB from natural deliveries exposed to labour-induced systemic inflammatory reaction, and in many reports UCB collection occured during planned caesarean section, in which a response to LAs could be different. Fourthly, our experiments were performed under normoxic conditions, while physiological hypoxia and acidosis in situ might have influenced cell reactivity.
Our study demonstrates no effects of LAs on the viability of UCB CD34+CD133– and CD34+CD133+ cell populations which may determine their transplantogenic value. The results obtained are important for both basic biology of these cells and also may have clinical implications. Our observations indicate that regional anaesthetic techniques for labour pain control and/or surgical delivery do not have negative impact on UCB cell survival and as such should not be of concern.



