Purpose
Brachytherapy is a highly precise radiation therapy technique that delivers a high-dose of radiation to tumors, while minimizing exposure to surrounding healthy tissues. This technique follows the inverse square law, ensuring rapid dose fall-off. It is commonly used for prostate and cervical cancers, but is also applicable to other malignancies, including head and neck cancer, rectal cancer, and gynecologic tumors, especially in cases requiring re-irradiation or palliative treatment [1-3]. Unlike external beam radiation therapy, brachytherapy requires direct implantation of applicators or needles into the tumor, making the procedure technically demanding. The accuracy of needle placement is critical to achieving optimal dose distribution while avoiding injury to surrounding organs. In complex cases, precise planning and multi-disciplinary collaboration are essential to ensure safety and efficacy. Recently, the integration of virtual reality (VR) and augmented reality (AR) technologies has gained attention in the medical field. These technologies have been utilized for surgical navigation, medical education, and procedural training, enabling practitioners to visualize and interact with 3D anatomical structures in a virtual environment [4, 5]. In brachytherapy, VR can enhance pre-operative planning, improve communication among specialists, and facilitate real-time guidance during needle insertion. Previous studies have reported the use of mixed reality and intra-operative holographic support in interventional procedures, demonstrating their potential to enhance precision and safety [6]. Furthermore, 3D printing has emerged as another innovative approach in complex surgical planning. By creating patient-specific anatomical models, 3D printing enables hands-on simulation and individualized treatment planning, particularly in challenging cases. While 3D printing provides tactile feedback and static visualization, VR offers a more dynamic and interactive experience, allowing real-time adjustments and collaboration among multiple specialists. The combination of these technologies may further enhance procedural accuracy in brachytherapy. In this case report, we described two patients who underwent brachytherapy with VR-assisted simulation, illustrating how VR technology improved pre-operative planning, inter-departmental collaboration, and procedural efficiency. Our experience highlighted the potential of VR to expand the accessibility of brachytherapy, particularly in non-routine treatment sites, where expertise may be limited. Both patients provided written informed consent for publication of their clinical data and images in this report.
Cases presentation
Case 1
A 51-year-old woman was diagnosed with carcinoma of unknown primary and poorly differentiated adenocarcinoma five years ago. She had a large tumor in the right common iliac region, extending into the internal and external iliac regions, and underwent definitive intensity-modulated radiation therapy (IMRT) with 60 Gy in 30 fractions. Three years later, she developed an in-field recurrence, for which brachytherapy with 42 Gy in 7 fractions was delivered to the ventral side of the treatment site. At the time of current presentation, the tumor in the right internal iliac lymph node region increased in size, invading the sacrum (Figure 1A, B), leading to severe pain. Due to prior radiation exposure, further external beam radiation therapy (EBRT) was deemed unsuitable, as the cumulative dose had reached tolerance limits of the internal iliac artery, vein, and bowel. Given this constraint, interstitial brachytherapy was selected as a localized treatment option.
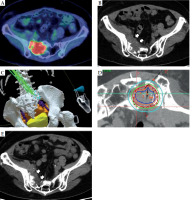
Fig. 1
A) Pre-treatment PET-CT showing abnormal uptake in the right internal iliac lymph node region with sacral invasion. B) Pre-treatment non-contrast CT with the tumor mass adjacent to the sacrum. C) Bone (white), bladder (yellow), bowel (orange), blood vessels (purple), and tumor (red) are contoured on Oncentra Brachy treatment planning software, which displays them in VR. Furthermore, an image of a needle (green) was created from the back of the lesion. D) Dose distribution diagram. 25 Gy in 5 fractions was used as the prescribed dose. The red dotted line represents gross tumor volume (GTV), while the solid red line signifies 100% dose. The blue line is 200% dose, the green line is 80% dose, and the light blue line is 50% dose. E) Follow-up non-contrast CT at 6 months post-treatment, showing a slight reduction in tumor size, as compared with pre-treatment imaging
VR simulation
To facilitate precise needle placement and effective inter-disciplinary collaboration, a VR-based simulation was performed. A 1 mm slice computed tomography (CT) scan was obtained, and radiation oncologist delineated the tumor, bones, blood vessels, bladder, and bowel using Oncentra Brachy treatment planning software (Elekta, Veenendaal, The Netherlands). These structures were exported in DICOM format and converted into STL files using 3D Slicer, an open-source medical image computing platform. The resulting models were then imported into Meta Quest 3 (Meta Platforms®, Menlo Park, CA, USA), enabling three-dimensional visualization of anatomical structures in a virtual reality environment. Using VR interface, the radiation oncologist and medical physicist jointly evaluated optimal needle positions, insertion angles, and depths based on the tumor’s location and its spatial relationship to adjacent critical structures (Figure 1C). This simulation allowed real-time interaction and multi-disciplinary discussion, leading to a consensus on the safest and most effective needle insertion strategy.
Treatment planning and execution
A preliminary brachytherapy treatment plan was generated in Oncentra, incorporating the VR-based findings. The radiation oncologist, IR physician, and medical physicist reviewed the plan to ensure optimal dose distribution while avoiding critical structures. Based on the pre-simulation results, the IR physician inserted ten 5-French ProGuide® sharp plastic needles (Nucletron, Elekta AB, Stockholm, Sweden) under CT fluoroscopy guidance. Needle insertion and treatment were completed without complications (Figure 1D). The prescribed dose was 25 Gy in 5 fractions.
Follow-up
At six months post-treatment, follow-up CT demonstrated a slight but measurable reduction in tumor size (Figure 1E), suggesting a response to brachytherapy. However, the pre-existing right lower limb lymphedema did not improve.
This case highlighted how VR-based pre-simulation facilitated inter-departmental collaboration, optimized treatment planning, and enhanced procedural accuracy, ultimately improving patient safety and treatment outcomes. The use of VR-based pre-simulation allowed radiation oncologists, interventional radiologists, and medical physicists to collaboratively determine the safest and most effective needle placement strategy. Multi-disciplinary collaboration has been recognized as a key factor in optimizing complex brachytherapy procedures, especially in cases requiring re-irradiation [7].
Case 2
A 46-year-old man was diagnosed with basal cell carcinoma of the oral cavity and squamous cell carcinoma (T3bN3bM0, stage IVB, UICC/AJCC TNM, version 8.0). He initially presented with a locally advanced tumor in the floor of the mouth, and was scheduled for total glossectomy and laryngectomy following neoadjuvant chemotherapy (NAC) (Figure 2A). However, after a favorable response to NAC, the patient chose radiotherapy over radical surgery.
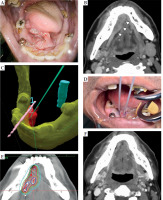
Fig. 2
A) Pre-treatment intra-oral image indicating a tumor in the right floor of the mouth. The lesion runs from the sublingual surface to the floor of the oral cavity on the right side of the lingual frenulum. B) Pre-treatment contrast-enhanced CT with a tumor in the right floor of the mouth adjacent to the mandible. C) VR-based simulation showing the relationship between the tumor, mandible, and planned needle positions. Mandible (yellow), tumor (red), spinal cord (light blue), and the needle embedded in the mouthpiece (pink) are contoured on the Oncentra Brachy treatment planning software, all of which are visible in VR. In addition, an image was created of a needle (green) being inserted into the lesion from two different directions. The positional relationship between the tumor, mandible, and needle is now more visible. D) Needle placement during brachytherapy. Needles were inserted between the sublingual surface and the floor of the oral cavity. Following that, mouthpieces were worn for treatment planning and execution. E) Dose distribution map for brachytherapy treatment planning. The red dotted line represents gross tumor volume (GTV), while the solid red line indicates 100% dose. The blue line denotes 200% dose, the green line – 80% dose, and the light blue line – 50% dose. F) Follow-up contrast-enhanced CT at 6 months post-treatment, demonstrating complete disappearance of the tumor
Radiotherapy and brachytherapy indication
Intensity-modulated radiation therapy (IMRT) to a total dose of 70 Gy in 35 fractions was scheduled. To ensure adequate tumor coverage while sparing the mandible and surrounding healthy tissues, a brachytherapy boost of 18 Gy in 3 fractions was added. The tumor, located adjacent to the mandible and oral mucosa (Figure 2A, B), posed technical challenges for needle insertion. Therefore, to minimize patient discomfort and avoid complications from severe mucositis, brachytherapy was planned early in the external beam course.
VR simulation
VR-based pre-simulation was performed to assist in accurate needle placement. A CT scan was acquired with a custom-made mouthpiece, designed to displace the tongue and protect adjacent mucosa. CT and contrast-enhanced magnetic resonance imaging (MRI) were fused using Eclipse (Varian Medical Systems, USA), while relevant structures, including the tumor, mandible, vascular anatomy, tongue, and mouthpiece, were segmented and visualized in VR (Figure 2C). This enabled collaborative planning of needle positions, angles, and insertion depths to optimize dose delivery and prevent critical structures.
Treatment planning and execution
Brachytherapy was delivered over three consecutive days. Needles were inserted under local anesthesia each day and removed after treatment. Needle retention was avoided due to difficulty of suture fixation in the oral floor and expected patient discomfort from prolonged insertion. A dentist managed anesthesia and tongue stabilization using the mouthpiece (Figure 2D, E).
Follow-up
The patient developed grade 3 (as per CTCAE v. 5.0) oral mucositis and candidiasis during the later phase of IMRT. Symptoms resolved within one month post-treatment. At six months, follow-up contrast-enhanced CT confirmed complete tumor disappearance (Figure 2F).
VR workflow
A standardized virtual reality (VR)-based simulation workflow was implemented in both the cases to support treatment planning and improve procedural safety. While case-specific details were described in the individual case sections, the general workflow was as follows:
First, thin-slice CT and contrast-enhanced MRI scans were obtained. The tumor and relevant anatomical structures, such as bones, blood vessels, bladder, bowel, and in case 2, a custom-made mouthpiece, were delineated using Oncentra Brachy (Elekta, Veenendaal, The Netherlands) or Eclipse (Varian Medical Systems, Palo Alto, CA, USA). The segmented structures were exported in DICOM format and converted into STL files using 3D Slicer, an open-source medical image processing software. These files were then visualized in Meta Quest 3 (Meta Platforms®, Menlo Park, CA, USA), facilitating immersive three-dimensional interaction within a virtual reality environment. Using this system, the radiation oncologist and medical physicist collaboratively reviewed spatial relationships, assessed optimal needle paths and insertion depths, and discussed the safest and most effective approach prior to procedures. This process enabled inter-disciplinary consensus and informed treatment planning.
Discussion
Brachytherapy is a highly effective treatment approach for various malignancies, especially in cases requiring dose escalation or re-irradiation. However, its technical complexity, including precise needle placement and avoidance of critical structures, often limits its accessibility. In this report, we demonstrated the successful application of VR-based pre-simulation to facilitate brachytherapy planning and execution in two challenging cancer cases.
Case 1: VR in multi-disciplinary collaboration and pre-operative planning
In case 1, the patient had undergone prior EBRT and interstitial brachytherapy, making further EBRT infeasible due to cumulative dose constraints. VR pre-simulation enabled a multi-disciplinary team, consisting of radiation oncologists, interventional radiologists, and medical physicists, to collaboratively determine the safest and most effective needle placement strategy. The ability to visualize the tumor, sacral bone, and surrounding organs in a 3D virtual environment helped optimize treatment planning and improved procedural accuracy.
Case 2: VR in training and procedural consistency
In case 2, the primary challenge was the technically demanding nature of interstitial brachytherapy in oral cavity cancer, requiring precise needle placement while avoiding the mandible, tongue, and critical vascular structures. VR pre-simulation allowed radiation oncologists, dentists, and medical physicists to share anatomical insights, simulate needle trajectories, and refine treatment strategy before the actual procedure. Additionally, because different operators performed needle insertions on separate days, VR provided consistent pre-operative references, ensuring uniformity in the procedure despite physician’s variability.
The expanding role of VR in brachytherapy
Over the past decade, augmented reality (AR) and virtual reality (VR) technologies have been increasingly integrated into medical education, surgical navigation, and procedural training. In the field of brachytherapy, previous studies have reported the use of mixed reality (MR) and intra-operative holographic support in interventional procedures, demonstrating their potential to enhance precision and safety [6]. Recent reviews have highlighted the potential of AR in interventional radiotherapy, particularly in real-time procedural guidance [7]. While AR can overlay holographic images onto the surgical field, VR enables pre-operative three-dimensional interaction, allowing clinicians to improve treatment strategies before the procedure.
Notably, Liu et al. reported a CT-MRI fusion-based 3D hologram technique for brain brachytherapy, permitting surgeons to visualize tumor structures intra-operatively, improving spatial orientation and treatment accuracy [8]. While MR offers real-time overlay of 3D structures onto the physical environment, VR provides an immersive, interactive simulation before the procedure. The integration of these technologies may further enhance brachytherapy precision. Additionally, mixed reality and intra-operative holographic support utilization have been reported for interventional procedures, suggesting a broader role for these technologies in enhancing real-time decision-making and operator guidance [7]. VR has shown potential in enhancing multi-disciplinary coordination, which is crucial for complex brachytherapy procedures. A recent study emphasized that high-complexity interventional radiotherapy benefits significantly from well-structured team collaboration and imaging-based procedural planning [8].
VR has shown to enhance proficiency and trainee confidence in brachytherapy procedures. A previous study reported that VR-based training improved engagement and procedural accuracy in gynecologic brachytherapy [9]. Similarly, in our case, VR pre-simulation allowed practitioners unfamiliar with head and neck brachytherapy to gain a better understanding of the anatomical constraints before performing the treatment. Brachytherapy remains underutilized in various malignancies due to limited expertise and challenges in accessibility [10]. VR technology might serve as a bridge to overcome these barriers, enabling practitioners to pre-experience the procedure in a risk-free environment before performing it on real patients. Mastering brachytherapy requires extensive training over time, and a previous survey identified significant gaps in interventional radiotherapy education [11]. VR-based pre-simulation may address these gaps by providing an immersive learning experience, especially for complex treatment sites. Compared with 3D printing, which provides static anatomical models for pre-operative planning, VR offers an interactive, real-time simulation environment, with dynamic adjustments and collaborative decision-making. Previous studies have shown that 3D-printed models improve tactile understanding of complex anatomical structures, making them useful for surgical training [12]. In neurosurgery, for example, patient-specific 3D-printed models have been utilized for pre-operative planning and education, allowing surgeons to gain a practical understanding of spatial relationships before the procedure [13]. Moreover, VR’s advantage of real-time interaction allow multiple specialists to review, manipulate, and modify treatment plans collaboratively before the procedure. A comparative study demonstrated that VR angiograms provided an immersive learning experience superior to 3D-printed angiograms, particularly in terms of spatial awareness and procedural rehearsal [14]. This suggests that while 3D printing is valuable for tactile learning and static pre-operative reference, VR provides a more dynamic and adaptable platform for procedural planning. As seen in our cases, VR can facilitate procedural consistency across multiple operators, making brachytherapy more accessible even in centers with limited expertise.
Future implications and limitations
Although this report highlighted the potential benefits of VR-assisted brachytherapy, several limitations should be acknowledged. First, VR pre-simulation does not yet offer real-time intra-operative guidance, which could further improve precision. Future advancements integrating VR with real-time imaging modalities (e.g., fluoroscopy, ultrasound, or MRI-guided brachytherapy) may enhance its clinical utility. Second, the effectiveness of VR in improving procedural outcomes should be validated through larger studies comparing VR-assisted with conventional brachytherapy approaches.
Despite these limitations, our findings indicated that VR technology can improve procedural accuracy, enhance inter-departmental collaboration, and expand the accessibility of brachytherapy to complex treatment sites. By incorporating VR-based pre-simulation into routine brachytherapy workflows, institutions can improve treatment safety, efficiency, and training for less experienced practitioners.
Conclusions
Virtual reality technology was successfully used for pre-simulation in two brachytherapy patients. In case 1, VR facilitated multi-disciplinary collaboration among radiation oncologists, interventional radiologists, and medical physicists, allowing optimized needle placement in a complex re-irradiation setting. In case 2, VR enabled inter-disciplinary planning and hands-on pre-simulation, which improved procedural accuracy and consistency among multiple practitioners, including radiation oncologists and dentists.
These findings suggest that VR-assisted pre-simulation can enhance procedural accuracy, facilitate knowledge sharing, and improve training for complex brachytherapy cases. By integrating VR into routine brachytherapy workflows, even treatment sites that previously required highly specialized expertise may become more accessible, ultimately expanding treatment options for patients. Future studies should explore the broader applications of VR technology in brachytherapy, including its integration with real-time imaging modalities for intra-operative guidance.



