Purpose
Endoesophageal brachytherapy (EBT) or endo-esophageal interventional radiotherapy (EIRT) is a very effective and widely accepted technique that is recommended in guidelines for the treatment of esophageal cancer in palliative setting and in medically inoperable cases [1-3].
This technique was first described as palliative treatment by Bartçat and Guised in 1909 using radium candles. In 1925, Guised et al. published first series showing excellent palliative results in 270 patients [4, 5]. Thereafter, in the late 20s, EBT became the preferred treatment in palliation. In the 50s-70s, external beam irradiation (EBRT) has been developed using Co60 γ-rays or X-ray from linacs, leading to underuse of esophageal brachytherapy. In the 70s, developments in brachytherapy (BT) included the extensive use of afterloading systems and different radionuclides, such as iridium-192 (192Ir), caesium-137 (137Ce), californium-252 (252Cf), and aurum-198 (198Au) [6]. High-dose-rate (HDR) sources using 192Ir were beneficial to patients to deliver treatment in short time with a high number of fractions, on an outpatient treatment basis. In 1976, Abe et al. reported the first HDR treatment for palliation in esophageal cancer [7]. A review of publications in PubMed database from 1980 to 2000s revealed 123 articles on this technique in patients treated with curative and palliative aims, and 130 papers thereafter. In the same period, 55,290 articles were found using the cross match of ‘esophagus treatment’, while with the search term ‘dysphagia palliation’, 1,743 articles were identified, with only 154 being related to esophageal BT. Based on these data, it could be assumed that this technique is not useful for patients, when in fact it is mainly a question of a lack of diffusion of the results using this technique.
The aim of the present manuscript was to emphasize the present role of EBT based on expert consensus opinion and data from the literature.
Why use brachytherapy in esophageal cancer?
Esophageal cancer is the 8th most frequent cancer in the world. Although it is less frequent in women, it is now on the rise in association with tobacco use. At present, adenocarcinoma is the most frequent cancer, with an increase in incidence of 350%. In addition, esophageal cancer mortality has increased 7% in the last 25 years, and since 2000, the 5-year survival is estimated as 19% [8]. The use of new targeted agents and immunotherapy may improve outcomes in the near future.
The elective treatment of esophageal cancer is surgery associated with radiochemotherapy, depending on the stage. However, the prevalence of this tumor in elderly and very elderly patients has increased, and surgery or chemotherapy is often not possible in these patients. EBT is a good therapy for dysphagia palliation as well as curative treatment. Several studies have demonstrated benefits in dysphagia palliation, with good 5-year survival rates in T1-2 cases not suitable for surgery and chemotherapy using EBT with HDR-BT [2, 3]. More recently, 125I seed coated expandable stents have been used in palliative care [9-11]. EBT administers high doses to the endoluminal component of tumor with a rapid fall-off, leading to a lower dose to normal surrounding soft tissues. It also allows a reduction in the overall treatment time, leading to consequent reduction in tumor cell re-population. Another benefit is that the tumor dose can be increased, particularly in patients not suitable for chemotherapy [12-20].
Other possible indications for EBT include [12-20]:
As a boost procedure prior to combination with radiochemotherapy. Thus, as an upfront procedure in selected cases, the nutritional status or operability of patient can be increased (cT2 and cT3).
In tumors in the thoracic esophagus as a boost to reduce the risk of radiation pneumonitis and chronic cardiac morbidity by delivering a reduced EBRT dose and lowering the mean heart and lung doses.
In cases of inadequate down-sizing following radiochemotherapy (< 70% reduction of the maximum standard uptake value in pre-operative fluorodeoxyglucose positron emission tomography re-staging).
To prevent re-obliteration of a stent (if esophageal fistula can be ruled out by computed tomography [CT]).
In local relapse after primary radiochemotherapy.
Various technical aspects
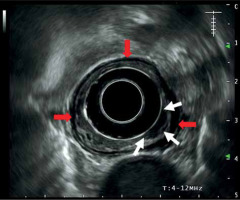
Although this review was not aimed at describing the technique, some aspects should be considered. EBT is an easy technique to perform, but requires some experience and infrastructure. The method for applicator placement is described elsewhere [12, 21]. Previous CT and ultrasonography are necessary to localize the tumor and neighboring organs at risk (OARs). Endoesophageal ultrasonography allows analysis of mucosa/sub-mucosal and intra-mural invasion as well as staging [12]. Figure 1 shows an ultrasonography of a T1 esophageal tumor.
Fig. 1
Esophageal ultrasonography demonstrating a hypo- echogenic thickening (white arrows) corresponding with T1 tumor extension with preservation of the muscularis layer (hypoechogenic line shown by the red arrow)
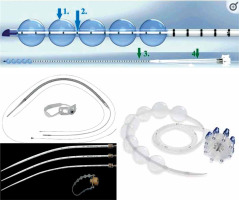
The applicator should be as large as possible in order to reduce the dose to the mucosa and maintain the applicator centered in the esophagus. High doses to the mucosa have been associated with an increase in complication, such as ulcerations, fistula, and stenosis. The use of a nasogastric tube as an applicator tube, can greatly increase the dose to the mucosa [12, 22]. There are also very useful transoral applicators (bougie-applicators), with a central canal fitting to the bronchus catheter diameter of most afterloading systems. Figure 2 demonstrates some of the different types of applicators.
All series in the literature report the use of treatment with 2D planning, although 3D planning is more accurate and allows better dose distribution, particularly to neighboring OARs. Figure 3 shows an example of a 2D planning treatment. Real-time personalized planning with 3D dosimetry can take 30-35 minutes, including radiation time with the patient sedated. When gastroscopy is performed in gastroenterology department, patient must be transferred to radiation oncology department, where CT scan can be performed quickly to diminish patient’s discomfort. There are two options to reduce the overall time for applicator placement plus CT image acquisition, and 3D planning and treatment: 1) After CT is performed, the applicator is removed and dosimetric analysis can then be accurately calculated considering OARs, such as the lungs, trachea, large vessels, or heart. The treatment is performed on other days, following the same plan, or 2) the first treatment is pre-calculated considering the length of planning target volume (PTV) and the patient is treated without 3D planning, followed by a 3D dosimetric analysis obtained from the CT planning for the next days. The discomfort induced by these options is likely the reason all the current studies have been reported using 2D planning, with very little data on dose constraints to OARs. Figure 4 demonstrates an example of the procedure and 3D treatment planning.
Fig. 3
Example of a 2D treatment. After the applicator was placed, 2 orthogonal X-rays were performed in these patients, including the length of the treatment. Dosimetric distribution was obtained afterwards
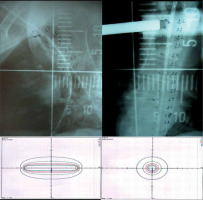
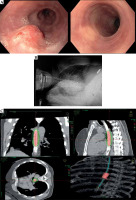
Fig. 4
Example of a 3D image-guided brachytherapy treatment in a patient with a T2 tumor. A) Tumor affecting the esophagus and response after 3 months of EBRT + BT treatment. B) The applicator was placed with endoscopic guidance at a determined position of dental arcades, and computed tomography with the applicator was performed and shown. C) Dose distribution in coronal, sagittal, and axial images
One of the main concepts to consider EBT is that esophageal cancer can differ in size and shape and consequently, the dose distribution may not be homogeneous. Moreover, hot and cold areas may occur due to patient’s movement and short time of treatment administration. In order to better optimize the treatment, the gross tumor volume, clinical target volume, and PTV require precise definition. Another aspect that should be mentioned is the need for better applicators that allows for larger diameters to reduce the dose to the healthy mucosa, facilitate centering of the applicator in the esophagus, and also for making the procedure more comfortable for the patient in case of a lack of anesthesia. At present, EBT should be performed with 3D-based planning, particularly in patients with curative intention, in order to reduce complications and obtain better tumor dose distribution. Magnetic resonance imaging (MRI) or endoscopic ultrasound-based target definitions should be the current standard [12].
The contraindications for EBT include stenosis obstructing endoscope passage (pediatric Ø 6 mm), non-tumoral stenosis after EBRT, life expectancy less than 8 weeks, tumors ≥ 10 cm in length, lack of patient’s co-operation, infiltration of trachea, bronchus, or large vessels, and medical contraindications to anesthesia or sedation. EBT in cervical esophagus or involvement of the cardia are not absolute contraindications. In cases with stenosis obstructing the passage of the endoscope, laser debulking prior to EBT can be considered [12].
Literature results
The use of HDR-EBT started in the 70s, leading to techniques and fractionation schedules, which continue to evolve to date.
EBT as exclusive treatment in T1-T2 tumors
Endoesophageal brachytherapy has been administered as exclusive treatment in inoperable T1-T2 tumors with good results in a few series. The most common schedule was 5 Gy × 6 fractions, prescribed at 5 mm from the applicator surface. The overall dose administered ranged between 25 and 48 Gy, with local control at 5 years between 50% and 100%. Low number of studies, each with a small population of patients, has made establishing a gold standard in exclusive EBT very difficult [3, 23-25].
EBRT + EBT in T1-T2 tumors
T1b tumors carry a 20% risk of mediastinal lymph node metastasis and, therefore, EBRT + EBT seems to be the most accepted treatment in cases of T1-T2 inoperable esophagus [2, 3, 12]. In the most relevant series after 1995 (shown in Table 1), patients received 2D-EBRT with a dose ranging from 45 to 66 Gy, followed by 2D-EBT of 2-4 fractions of 8 to 24 Gy, prescribed at 5 mm from the applicator surface. The applicator diameter ranged between 10 and 20 mm, and the disease-free survival (DFS) varied between 55% and 85%, increasing to 97% for superficial tumors. Late complications were related to the EBT dose and the number of fractions with 17% for 12 Gy vs. 80% for 24 Gy. Ulcerations appeared in 3.5% to 20%, depending on the series, and less frequent complications included fistula, esophageal stenosis, pneumonitis, and cardiac problems (2 deaths from pneumonia after developing fistula were reported). Before 1995, different studies reported varying local control rates using EBT doses ranging from 12 to 40 Gy in 1-3 fractions regimen, with only one 5-year series showing a local response rate of 64% and overall survival of 18%. In stage I, the overall survival at 1-year ranged between 26% and 78%, and in stage II, it ranged between 21% and 26%. Late complications included ulceration in 10-100%, stenosis in 7-42%, and fistula in 3-9% of patients [26-30]. The use of higher doses, which was associated with a higher complication rate before 1995, may have led to underusage of this technique. However, it should be taken into account that independently of the radiation format chosen (low-dose rate or HDR), EBT is a very useful technique in T1-T2 inoperable esophagus, and some technical aspects have been learned over time: the applicator diameter should be as large as possible, with 2 fractions of 5 Gy HDR being adequate after EBRT, and the results are better in superficial tumors. After 1995, the 5-year DFS was reported between 54% and 85% [14, 31-37]. In one report by Murakami et al., the cancer-specific survival at 5 years was 97% for superficial tumors and 55% for submucosal tumors [32].
Table 1
Retrospective results of endoesophageal brachytherapy in T1-T2 tumors
| Author(s), year [Ref.]. | n | T (TNM) | EBRT (Gy) | BT (Gy) | Fraction number | 100% dose @ (mm) | Applicator’s diameter | Chemotherapy | Complete response (%) | Local control | DFS | Complications | Remarks |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yoruzu et al., 1999 [31] | 124 | T1-2 | 40-60 | 8-24 | 2-4 | 5 | 10-15 | Yes (41%) | 73 | 1 y: 68%, 2 y: 58% T1: 73%, T2: 44% | – | 12 Gy: 17% 24 Gy: 80% | LC: ≤ 15 Gy: 42%; > 16 Gy: 59% |
| Murakami et al., 1998 [32] | 32 | T1-2 | 50-66 | 10-12 | 2 | 5 | Yes (32%) | 1 y: 100 | 1 y: 85-83% 2 y: 70-83% | LC and DFS: T1 = T2 | Ulcers: 8/32 (20%) | CSS: 2 y: T1: 100%, T2: 93% | |
| Okawa et al., 2000 [34] | 43 | T1-4, N0-1 | 60 | 10 | 2 | 5 | 10 | Yes | 56 | – | DFS at 2 and 5 y: < 5 cm: 76.4% and 64%; > 5 cm: 39.4% and 31.5% | 1 stenosis 1 cardiac | |
| Pasquier et al., 2006 [35] | 66 | Superficial | 57.1 | 7 | 2 | 5 | 13 | Yes | 98 | – | 3, 5 and 7 y: 63%, 54%, and 54% | 9% | |
| Yamada et al., 2006 [36] | 63 | T1 | 55-59.4 | 12 | 2-3 | 5 | 15 | Yes | – | 5 y: 63.7% T1a 84.4%, T1b 50% | 3 ulcers or stenosis (10%) | ||
| Ishikawa et al., 2010 [14] | 36 | T1 | 60 | 10-9 | 2-3 | 5 | 10-20 | Yes | 87 | – | 5 y: 59% | 6 (20%) | Better EBRT + BT |
| Tamaki et al., 2012 [26] | 54 | T1 | 56-60 | LDR: 10 HDR: 9 | LDR: 2 HDR: 3 | 5 | 15-20 | No | 80 | 81% | 5 y: LDR 83%, HDR 84.9% | 2 deaths (pneumonitis by fistula) | LDR similar to HDR |
| Murakami et al., 2012 [33] | 87 | T1 | 45-46 | 10-15 | HDR | 5 | 16/20 | No | 98 | 5 y: Superficial 75%, Sub-mucosal 49% | – | 4 pneumonitis, 10 cardiac, 2 fistulas, 3 ulcers | CSS at 5 y: Superficial 97% Sub-mucosal 55% OS: Superficial 84% Sub-mucosal 31% |
A more recent approach delivered EBRT + EBT after incomplete endoscopic resection of T1-T2 tumors. A study with 37 patients by Nishibuchi et al. described a 5-year DFS of 64%, and an overall survival of 78% [38].
EBRT + EBT in advanced tumors
Advanced stages of esophageal cancer have been treated with EBRT + EBT, with 2-year survival rates ranging between 17% and 34%, and from 10% to 28% at 5 years. Table 2 presents the most relevant series of EBRT + EBT for advanced cases, with late complication rates for ulcerations of 3-11%, 8-16% for stenosis, 9% for fistula, and 5% for fatal hemorrhage [21, 39-45]. These complications, especially the latter two, may also appear in patients with tumor reduction after chemotherapy and EBRT, or in tumor progression. The lack of benefit in increasing overall survival can be explained by the heterogeneity of these tumors and high probability of distant metastasis, although an increase in local control has been described by Someya et al. in tumors < 5 cm compared with tumors ≥ 5 cm [43]. Studies evaluating local control, dysphagia-free time, and quality of life are needed. These combined treatments have been performed using 2D or 3D planning for EBRT, and EBT has been administered after 2D planning. The most accepted schedule in these patients is 2 fractions of 5 Gy using the largest applicator diameter. EBT should be considered when there are contraindications for chemotherapy.
Table 2
Retrospective results of endoesophageal brachytherapy in advanced stages
| Author(s), year [Ref.] | n | Stage | EBI (Gy) | BT (Gy) | Fraction number | Applicator’s diameter (mm) | 100% dose @ (mm) | Chemotherapy | Response | OS (y) | Complications | Remarks |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hujala et al., 2002 [40] | 40 | III-IV | 40 | 10 | 4 | 6 | 10 | No | – | 1 y: 30% 2 y: 17.5% | – | – |
| Vuong et al., 2005 [39] | 70 | T1-3, N0-N1 | 50 | 20 | 5 | – | 5 | Yes | LC 2 y: 75% | 5 y: 28% | – | 2 y: 25% local relapse, 28% M1 |
| Lopez et al., 2007 [59] | 23 | II-IV | 44.2 | 21 | 5 | 6-10 | 7 | No | CR: 23% | 5 y: 10% | No | 5 y OS: BED > 33 Gy: 16.67%, < 28 Gy: 0% |
| Muijs et al., 2011 [41] | 62 | T1-T4, N0-1, M0-1 | 40 + 20 | 12 | 2 | 6 | 10 | No | LC 1 y: 71% 2 y: 50% 3 y: 45% | 1 y: 57% 2 y: 34% 3 y: 11% | Ulcers: 11% Stenosis: 16% | Excessive toxicity |
| Calais et al., 1997 [16] | 53 | IIB-III | 60 | 10 | 5 | 10-14 | 5 | Yes | LC 1 y: 74% 3 y: 30% | 3 y: 27% | Stenosis: 13% Severe late toxicity: 11% | |
| Taal et al., 1996 [42] | 51 | III-IV | 40 | 10 | 5 | 6 | 7 | No | CR: 60% PR: 40% | 1 y: 20% | Ulceration: 6.6% Fistula: 8.8% Fatal hemorrhage: 4.4% | |
| Someya et al., 2002 [43] | 77 | III-IV | 40-65 | 10-24 | 2-3 | 10 | Mucosa | No | LC 2 y: < 5 cm: 83%, ≥ 5 cm: 25% | 2 y: 35.6% 5 y: 10.6% | – | Overall series with 100 patients stage I-IV: 2% stenosis and 1% dead by pneumonitis |
| laskar et al., 2015 [44] | 75 | III-IV | 20-30 | 16 | 2 | 6-8 | 5 | No | – | 1 y: 27% | 27% (stenosis, fistula, bleeding) | |
| Aggarwal et al., 2015 [22] | 59 | I-IV | 27-30 | 10-15 | 1 | 16-gauge | 10 | Not usual | – | 1 y: 51% 2 y: 19% 3 y: 7% | Stenosis: 8% Ulcers: 3% | |
| Kissel et al., 2020 [45] | 41 | III-IV | 30 | 15 | 3 | 13 | 5 | Not simultaneous | 50% | 1 y: 68% 2 y: 50% | – |
After the appearance of the CROSS trial in advanced patients in 2004, a tumor boost after 45-50 Gy radiotherapy plus chemotherapy using EBRT or BT has been largely discarded [46]. The role of dose escalation in advanced patients is being investigated afresh in the Concorde trial, in which patients received FOLFOX chemotherapy + 50 Gy EBRT, and were then randomized to receive an EBRT boost of 10 Gy vs. 26 Gy. The results of this study presented in an abstract did not show positive results favoring a tumor boost after EBRT [47].
EBT in palliation
Palliative treatment of esophageal cancer covers a very heterogeneous group of patients with varying tumor size and extent, patients’ characteristics, and life expectancy. Palliative treatment is mainly centered on the relief of dysphagia, although it may provide benefits in hemorrhage, pain, weight loss, and quality of life. EBT is a very effective treatment for dysphagia palliation, achieving palliation in 70-90% of the cases, with 50% of complete endoscopic response and a dysphagia-free time usually ranging between 2 and 9 months, depending on the series. In responders, even the simplest 2D HDR-EBT prolongs survival [48]. EBT is also a very useful treatment in patients with a relapse following EBRT [3, 49-51].
In palliative EBT, the dose and number of fractions should be adapted to the performance status of patient, previous radiotherapy doses, and life expectancy. Patients with a longer life expectancy may benefit from a higher number of fractions and higher overall dose, while those with a short life expectancy may benefit from a single-fraction of 10-15 Gy. Different equivalent dose to a schedule of 2 Gy per day related to dose per fraction and number of fractions is presented in Table 3.
Table 3
Equivalent dose to 2 Gy fraction depending on fractionation schedule
| Fractionation schedule | EQD2(α/β=10) (Gy) | EQD2(α/β=3) (Gy) |
|---|---|---|
| 1 × 10 Gy | 16.6 | 26 |
| 1 × 12 Gy | 22 | 36 |
| 2 × 8 Gy | 24 | 35.2 |
| 3 × 6 Gy | 24 | 32.4 |
| 3 × 7 Gy | 30 | 42 |
| 3 × 7.5 Gy | 32.8 | 47.2 |
In a study by Burchardt et al., 92 patients were treated for palliation of dysphagia, with 3 fractions of 7.5 Gy prescribed at 5 mm from the applicator surface. Good dysphagia palliation was achieved with an overall survival of 30% at 1-year among responder patients, compared to 10% in those with partial or no response. The results were better for adenocarcinoma types, and 5% of patients remained alive for more than 2 years [48]. While EBT is an easy technique and is currently recommended by the European Society of Gastrointestinal Endoscopy as a good technique for palliation, it requires infrastructure and experience, which are not available in many centers [51]. In 2002, the first randomized trial comparing stent and EBT (one fraction of 12 Gy) in the Netherlands, showed benefits for EBT in comparison to stent in dysphagia-free time, dysphagia palliation, weight loss, and improved quality of life [20]. Despite the positive results of this trial, up to 2017, few changes in practice occurred. Subsequently, in the same country, POLDER-1 trial was started using EBRT, with 4 fractions of 5 Gy in metastatic esophageal cancer patients with dysphagia (EQD2α/β=10 = 23.3 Gy). The results of the POLDER trial (2016-2019) were recently compared with those of SIREC trial (1999-2002), in which EBT was administered with one fraction of 10 Gy (EQD2α/β=10 = 16.6 Gy). This study concluded that both treatments offered similar results in dysphagia palliation at 3 months, but EBRT was better for controlling pain, nausea, vomiting, and loss of appetite. These benefits in palliation with EBRT could be considered as the result of a higher radiotherapy dose in the POLDER trial [52]. The very few studies available comparing EBRT and EBT used 10 fractions of 3 Gy (EQD2α/β=10 = 32.5 Gy), leading to more acute side effects in comparison with one fraction of EBT using a dose of 12 Gy [53-55]. Better dysphagia palliation has been reported in EBRT with 39 Gy in 13 fractions (EQD2α/β=10 = 42.5 Gy) in comparison to other lower dose EBRT schedules [54].
Two systematic reviews and meta-analyses have shown superior results for EBT in dysphagia palliation compared to other palliative techniques [55, 56]. A benefit from EBRT + EBT in combination has been demonstrated with respect to life expectancy and symptoms [57]. In view of the heterogeneity of patients with esophageal cancer requiring treatment for the palliation of dysphagia, each patient should be considered individually and receive treatment adapted to the patient’s needs. Treatment with EBRT is easier to perform compared to EBT; however, the choice between EBRT and BT should not depend on the availability of the technique, but rather the most appropriate technique and dose depending on patient’s characteristics and wishes.
Another technique that should be mentioned is seed coated stents for dysphagia palliation [9-11]. In particular, the results of a meta-analysis by Zhao et al. showed longer stent patency and survival than normal stent insertion [9].
Current studies in the pipeline on the palliation of dysphagia as an endpoint include: 1) The ROCS trial (NTC01915693): Phase III study analyzing the results of the use of stent ± EBRT; 2) The EXTENT trial (NTR 7116): Phase III study comparing stent vs. EBRT 20 Gy/5 fractions; and 3) The malignant dysphagia trial: Phase II study analyzing the benefits of stent ± BT 10 Gy. It is disappointing that these trials overlook the previously published results on the use of stents vs. EBT and EBT + EBRT doses, and it seems unlikely that they would contribute to defining optimal palliation of dysphagia in this patients’ population.
Why is a very easy, useful, old technique underused?
Endoesophageal brachytherapy is a very useful technique that provides palliation and improved survival, with an acceptable low-rate of complications in inoperable T1-T2 esophageal cancer patients. Late effects associated with the treatment appear after 6 months, with asymptomatic ulceration being the most common, and severe complications, such as stenosis, hemorrhage, or fistula occurring in only 5-10% of cases [2, 3, 12, 56].
EBT is a very effective treatment in the palliation of dysphagia; a randomized trial has demonstrated better results than stent placement, providing level 1 evidence [20]. Predicted DFS values based on radiation dose estimated with meta-regression models, indicate that the results are associated with dose, showing better results for 7 Gy × 3 fractions [57, 58].
The American Brachytherapy Society published its’ recommendations for EBT in 1997 [59]. Over the years, this effective technique has been maintained in several centers working in BT, treating significant numbers of patients per year. However, the use of this technique is not common, being reported as 2% of all BT treatments in Europe in 2007, and a national survey in Belgium reported that this value has not increased since then [60]. Several reasons discussed below may explain the underuse of this technique.
While EBT is a very easy technique to perform, it needs some infrastructure for patients’ management, which is why the technique is only available in established BT departments. Moreover, knowledge of the technical aspects and experience in patients’ management as well as the development of possible complications are all necessary. This is challenging when small numbers of patients are treated per center per year. Also, it should be considered that inoperable patients referred to BT departments are usually over the age of 60 years, and often have severe comorbidities usually related to the liver, heart, and lung diseases, and thereby require more complex supportive care.
Therefore, EBT is usually available only in large centers, and there is limited reporting of results and comparisons of performance, leading other centers to use different techniques, including stent placement, EBRT, chemotherapy, and endoscopic resection in inoperable patients, who require palliative treatment [61].
Another important aspect to consider is the presence of competition, not only with other medical specialties, such as surgeons, gastroenterologists, endoscopists (close collaboration between endoscopists and gastroenterologists is essential; the lack of motivated gastroenterologists hinders the development of EBT), but also with radiation oncologists, who usually ‘prefer’ to treat patients with EBRT. The fact that the results described in the literature before 1995, in which 2D planning was used in both EBRT and EBT reported a higher incidence of complications than with current techniques, has also encouraged the use of approaches other than EBT for palliative and curative treatment in inoperable patients.
Undoubtedly, EBRT is simple and widespread technique, while EBT is not available in most centers, and therefore is not used to its’ maximum potential. Furthermore, there is a lack of meta-analyses and dissemination of the results of EBT in comparison with other BT treatments.
How can we overcome underuse?
To overcome the underuse of EBT, analysis and diffusion of institutional results are necessary as well as meta-analyses of retrospective data and analysis of multicenter results. The justification for treatment, including the weight of the benefits and risks or complications, cost-effectiveness and aspects, such as patient’s preference that lead to underuse of EBT, should be demonstrated. Treatment should be adapted to individual patient’s characteristics when using intensity-modulated radiotherapy/volumetric arc therapy planning for esophageal EBRT and 3D planning for EBT, with proper number of applicators and doses. All the above would allow the design of prospective multicenter trials in cooperative groups. Additionally, centralization of the treatment in highly specialized departments might provide an impulse to the implementation and popularity of EBT treatment.
Conclusions
Endoesophageal brachytherapy is a very useful technique that offers good results in inoperable T1-T2 tumors and in the palliation of dysphagia. The patients must be adequately selected; the technique is easy and offers good results considering quality of life and duration of dysphagia palliation compared to laser or chemotherapy. Due to its’ limited availability, it is not being used to its’ maximum potential. As a result, in the absence of well-designed prospective studies on the use of EBT, the application of exclusive EBRT has increased. Taking all of the above into account, the use of EBT in esophageal cancer should be reconsidered in order to establish its’ real role in patients who can most benefit from this treatment.