Introduction
Osteoarthritis (OA), the most prevalent form of joint disease, causes disability in adults by inducing pain and impaired joint function. Osteoarthritis is characterized by pain, stiffness, locomotor restriction, bony enlargement, and sometimes swelling of specific joints such as those in the knees, hips, hands and spine, which contribute to impaired function [1-3]. The exact mechanisms involved in the pathogenesis of OA are not fully understood.
Synovial tissues mainly consist of synovial fibroblasts and macrophages, both of which play a pivotal role in synovial inflammation [4-8]. Macrophages, in particular, are central to synovial inflammation through their production of inflammatory cytokines [7, 9-13]. Macrophages also produce various matrix metalloproteases (MMPs) [11, 14-16]. Investigating the factors that regulate synovial macrophage activation could be important for the development of OA treatments.
β2-microglobulin (B2M) is a component of class I major histocompatibility complex (MHC) located on the surface of nucleated cells [17-21]. Evidence suggests that B2M fibrils found in synovial tissue may contribute to osteoarticular complications in patients undergoing chronic hemodialysis (HD) with dialysis-related arthritis (DRA). Given that OA patients have high B2M concentrations in their synovial fluid [22], B2M may also stimulate MMP-1 expression in synovial fibroblasts in vitro [23]. Several studies have reported that B2M stimulates inflammatory cytokine production in the human embryonic kidney epithelial cell line HEK293 and murine monocyte cell lines in vitro [24]. However, the effect of B2M on MMP and inflammatory cytokine production in human primary synovial macrophages remains unclear.
Here, we investigated the effect of B2M on the expression profiles of inflammatory cytokines and MMPs in human synovial macrophages derived from the osteoarthritic synovium.
Material and methods
Patients
Six women with an age range of 60-78 years [mean ± standard deviation (SD), 68.3 ±5.8 years] with radiographic knee OA (Kellgren/Lawrence grade: KL3 = 5/6, KL4 = 1/6) who received total knee arthroplasty (TKA) at Kitasato University Hospital were enrolled in the study. During surgery, synovial tissue was extracted from the participants’ operated knee. All six women provided informed consent the day prior to surgery. The study protocol was approved by the Ethics Review Board of Kitasato University (reference number: KMEO B13–113).
Extraction of synovial CD14-positive cells
Patients’ synovial tissues were treated with 20 ml of 0.1% type I collagenase from Clostridium histoluticum (Wako Pure Chemicals, Osaka, Japan) for one day at 37°C. Isolated mononuclear cells were then incubated with 0.5 ml of biotin-conjugated anti-CD14 human monoclonal antibody (BioLegend) for 30 min at 4°C, before washing twice in phosphate-buffered saline (PBS). The cells were subsequently exposed to streptavidin-conjugated magnetic beads (BD Biosciences) and moved to a cell separation system (IMag; BD Biosciences) where they were left to stand at 4°C. Following a period of 30 min, the cells were removed from the magnetic support. To extract CD14+ cells, 5 ml of prewarmed (37°C) α-minimum essential medium (MEM) was added before centrifuging the cells at 280 g for 5 min. After trypan blue staining, CD14+ cells (1 × 105 cells/5 ml) were seeded in a 6-well culture plate.
Synovial cell culture
Synovial macrophages were maintained in culture for one week in medium containing 100 ng/ml macrophage colony-stimulating factor. A previous study reported that bovine B2M is present in bovine serum and is able to replace endogenous B2M associated with several class I antigens from human and mouse cells maintained in culture [17]. To reduce the effect of fetal bovine serum (FBS)-derived bovine B2M, cells were subsequently incubated in α-MEM containing 1% FBS and 1% penicillin/streptomycin for 3 h before culturing for 6 and 24 h in the presence of 10 µg/ml recombinant human B2M derived from Escherichia coli (Oriental Yeast Co., Tokyo, Japan). We confirmed that cell viability was not affected in this condition using the WST-8 assay. A previous study reported that the B2M concentration was 2.32 ±0.72 µg/ml in the synovial fluid of severe OA patients [22]. However, in in vitro conditions, 1 µg/ml B2M had no effect, while 10 µg/ml B2M inhibited chondrocyte growth and enhanced the effect of MMP-1 on synovial fibroblasts [22, 23]. Therefore, we used 10 µg/ml B2M to stimulate macrophages in this study. The remaining cells were used as controls. To determine the time points, we evaluated the gene expression of macrophage markers, inflammatory cytokines, and MMPs at 1, 3, 6, and 24 hours after B2M stimulation in a few samples. Because we observed increased gene expression at 6 and 24 hours, we cultured synovial macrophages for 6 and 24 h in α-MEM supplemented with 1% FBS and 1% penicillin/streptomycin only in this study.
Real-time PCR
Synovial macrophages were homogenized with TRI-zol Reagent with a homogenizer. After centrifugation, the supernatant was obtained and total RNA was extracted using Direct-zol RNA Micro Prep (Zymo Research, Irvine, CA) based on the manufacturer’s protocol. The SuperScript III First-Strand Synthesis Super Mix kit (Life Technologies, Thermo Fisher, Waltham, MA) was then used to conducted cDNA synthesis with the purified total RNA as the template. Table 1 lists the primer sequences adopted in this study. To examine macrophage marker (CD80, CD163 and CD206), inflammatory cytokine (IL-6, IL-8 and TNFA)] and matrix metalloproteinase (MMP-9 and MMP-13) mRNA expression, real-time PCR analysis (MiniOpticon, Bio-Rad, USA) was conducted on a PCR Master Mix (Takara SYBR PreMix Ex Taq II). Expression was normalized to GAPDH mRNA according to the delta-delta CT method.
Table 1
Sequences of primers used in this study
ELISA
To analyze the effect of B2M on IL-6, IL-8, TNF-α and MMP-13 production, we conducted enzyme-linked immunosorbent assay (ELISA) on the culture supernatant using a commercially available kit (LEGEND MAX Human ELISA Kit, BioLegend, San Diego, CA, USA) based on the manufacturer’s protocol.
Statistical analysis
Statistically significant differences between variables were determined using a paired t test or the Wilcoxon signed-rank test. Whether or not the data were normally distributed was ascertained using Shapiro-Wilk’s test. Variables that were found not to be normally distributed were analyzed using the Wilcoxon signed-rank test between vehicle and B2M groups at each time point. Statistical significance was indicated by p < 0.05. All analyses were performed with SPSS software v19.0 (IBM, USA).
Results
Real-time PCR analysis
Stimulation of synovial macrophages with B2M for 6 h caused mRNA expression of CD80 to significantly rise compared to that observed in vehicle control cells (p = 0.005, Fig. 1A). On the other hand, stimulation with 10 ng/ml B2M for 24 h caused CD163 mRNA expression to become significantly reduced relative to vehicle control cells (p = 0.028, Fig. 1B). Meanwhile, 24-h and 6-h stimulation had no effect on CD80 and CD163 mRNA expression, respectively (CD80, p = 0.917, CD163, p = 0.084; Fig. 1A, B). Likewise, 6-h (p = 0.225) and 24-h stimulation (p = 0.345) with B2M had no significant impact on CD206 mRNA expression (Fig. 1C).
Fig. 1
Macrophage marker, inflammatory cytokine and MMP mRNA levels in synovial macrophages following exposure to β2-microglobulin (B2M). mRNA expression of A) CD80, B) CD163, C) CD206, D) IL-6, E) IL-8, F) TNFA. *p < 0.05 G) MMP-9 and H) MMP-13 in synovial macrophages after stimulation with B2M for 6 and 24 h. *p < 0.05

Stimulation of synovial macrophages with B2M for 6 h caused a significant increase in IL-6, IL-8 and TNFA mRNA levels compared to vehicle control cells (IL-6, p = 0.028, IL-8, p = 0.005, TNFA, p = 0.002; Fig. 1D-F). Similarly, stimulation for 24 h significantly elevated IL-6, IL-8 and TNFA mRNA expression relative to the vehicle control (IL-6, p = 0.028, IL-8, p = 0.028, TNFA, p = 0.046; Fig. 1D-F).
Stimulation with B2M for 6 h also significantly elevated MMP-13 mRNA expression compared to vehicle control cells (p = 0.004; Fig. 1H). In contrast, 24-h stimulation had no effect on MMP-13 mRNA expression (p = 0.917; Fig.1H). Similarly, MMP-9 mRNA expression did not change following 6-h or 24-h stimulation with B2M (p = 0.753, and p = 0.123, respectively; Fig. 1G).
ELISA
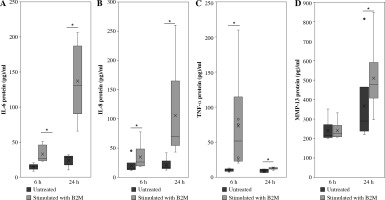
Stimulation of synovial macrophages with 10 ng/ml B2M for 6 h significantly elevated IL-6, IL-8 and TNF-α protein levels compared to vehicle control cells (IL-6, p = 0.013, IL-8, p = 0.028, TNF-α, p = 0.028; Fig. 2A-C). A similar increase was observed following 24 h of stimulation (IL-6, p = 0.007, IL-8, p = 0.028, TNF-α, p = 0.028; Fig. 2A-C). While MMP-13 protein levels were also significantly higher following 24-h stimulation with 10 ng/ml B2M (p = 0.028; Fig. 2D), no changes were observed after 6-h stimulation relative to vehicle control (p = 0.917; Fig. 2D).
Discussion
Macrophages respond to stimuli in their microenvironment by altering both their phenotype and function [25-27]. The synovium of OA patients harbors “M1” classically activated and “M2” alternatively activated macrophages, which are distinguished through their differentially expressed receptor and cytokine profiles [13, 25, 28, 29]. M1 macrophages predominantly express CD80 and produce inflammatory cytokines such as TNF-α, IL-6 and IL-8. In contrast, M2 macrophages preferentially express CD163 and CD206 [25, 30-32]. Further, the M1/M2 ratio has been shown to have a significant positive correlation with knee OA severity according to patients’ Kellgren-Lawrence grade [28]. Here, B2M promoted CD80 expression and increased TNF-α, IL-6, and IL-8 production, but decreased CD163 levels. A previous study reported B2M-mediated TNF-α production in the murine RAW 264.7 monocyte cell line [33]. Further, CD206+ M2-like macrophages have been shown to be increased in platelet-specific β2M knockout mice compared to wild type mice [34]. Taken together, our findings and those of previous reports suggest that B2M promotes synovial inflammation and contributes to OA pathology by promoting M1 polarization and inhibiting M2 polarization.
In addition to inflammatory cytokines, activated macrophages in the OA synovium secrete MMPs, which lead to cartilage degradation [9, 35-37]. Here, B2M increased MMP-13 mRNA expression at 6 h and MMP-13 protein production at 24 h in synovial macrophages. Therefore, stimulation with B2M temporarily increased mRNA expression, which led to subsequent protein synthesis. The lack of an earlier increase in MMP-13 protein may be due to the fact that protein synthesis takes time; hence, transcript changes may not have been reflected in protein levels at 6 h. Previous studies have indicated that macrophage polarization alters the MMP expression profile. MMP-9 and MMP-13 are dominantly expressed in M2 and M1 macrophages, respectively [11, 14-16]. Therefore, together with findings from other reports, our results suggest that increased MMP-13 may be associated with M1 polarization. Clinical reports have demonstrated increased MMP-13 levels in patients showing articular cartilage destruction, implicating high MMP-13 levels in cartilage degradation [38]. Further, a prior study demonstrated that transgenic mice with overexpression of Mmp-13 harbored several features of OA, including cartilage degradation [39]. B2M may thus contribute to cartilage destruction by increasing MMP-13.
A major strength of the present study was the use of primary human synovial macrophages derived from OA patients. However, there were also several limitations. First, synovial macrophages isolated from OA patients, even in the absence of stimulation with B2M, could exhibit basal pre-activation due to the presence of high levels of other damage-associated molecular patterns present in the inflamed joint. Further investigation using primary human macrophages (i.e. macrophages differentiated from the blood monocytes of healthy donors) is needed to confirm our findings. Second, we only studied the effects of a single dose of B2M. Finally, the mechanism by which B2M affects synovial macrophage polarization remains unclear.
In summary, B2M increased M1 macrophage marker, inflammatory cytokine, and MMP-13 expression in synovial macrophages. B2M-mediated activation of synovial macrophages may thus be associated with OA pathology.