Introduction
Celiac disease (CD) was previously considered a childhood disease that causes malabsorption and various digestive problems that dramatically affect children’s development, and it has a huge impact on their cognitive and developmental milestones. It is now known to be an immune-mediated disease that can affect any age group, and it has genetic characteristics that make some individuals more susceptible than others. It is triggered by the ingestion of gluten-containing products such as bread, barley, food pastes, and wheat [1].
In clinical practice CD frequently goes underdiagnosed due to its wide clinical presentations in children that could mimic any other disease of the endocrine system, or maybe due to its early exclusion from the differential diagnosis, especially in populations where its prevalence is not high. On the other hand, delayed onset of CD around the age of 5–7 years might be presented with uncommon gastrointestinal complaints (e.g. vomiting, recurrent abdominal pain, nausea) or other non-gastrointestinal presentations, such as iron deficiency, delayed puberty, dental enamel deformities, and short stature [2]. What is considered as a real challenge is the diagnosis in cases that only present with short stature as the main symptom [3]. Some works of literature on CD have described patients with short stature and CD, low levels of somatomedin, and normal release of growth [4]. Nevertheless, cases of growth hormone deficiency associated with CD are also reported [5]. The pituitary gland may well be a target for the autoimmune process in celiac disease [6]. Children with hypopituitarism and short stature associated with CD are not yet well-explained in the literature, the goal of this study was to briefly assess this relationship.
Written informed consent was collected from our patient and his caregiver (mother). This case report was approved by Kazan Medical State University and the Republican Children’s Clinical Hospital Medical Ethics Committee.
Case presentation
Patient
A male child, who was born from the second pregnancy of apparently healthy parents with irrelevant family history, and whose brother is a healthy male. The father’s height is 168 cm and the mother’s is 154 cm. Since his early childhood, the patient developed symptoms of bloating, abdominal pain, and discomfort. When the family started to notice his lag of growth behind his peers at the age of 9 years old, they turned to a paediatrician, who subsequently referred them to the endocrinology department of the Republican Children’s Clinical Hospital of Kazan, Russia. Examination and laboratory analysis determined the diagnosis of “hypopituitarism and growth hormone deficiency”.
Physical examination and laboratory testing
Upon examination, the child’s height was 113 cm (less than the third percentile for a 9-year-old), his weight was 20 kg (less than the third percentile for a 9-year-old).
According to clinical and laboratory studies, the following deviations were detected (Table I).
Table I
Laboratory and radiology values during inpatient evaluation (first visit at the age of 9 years)
Growth hormone stimulation tests
In this study, we used 2 standard provocation tests, insulin, and oral clonidine. In the test, clonidine (Clophelin®, Russia) was administered orally at a dose of 0.15 mg/m2. Blood samples were withdrawn before administration of Clonidine and 30, 60, 90, and 120 min after administration of the medicine (respectively). In a hypoglycaemic test, short-acting insulin (Actrapid®, Novo-Nordisk, Denmark) was administered intravenously at a dose of 0.1 IU/kg. Blood samples were withdrawn before the injection of insulin and 15, 30, 45, 60, 90, and 120 min after administration of the medicine (respectively); at the same time, the blood glucose level was carefully monitored.
Determination of the concentration of growth hormone in the blood serum was carried out using standard sets from the «Hema-Medica» company using the ADOL (immunofluorescence assay) method in the laboratory of the Republican Children’s Clinical Hospital of Kazan, Russia, which is certified to conduct these laboratory assessments. A comparison of the results was performed following the instructions for the test kits and analysed within the established range of normal concentration of hormones (Table II).
Interventions
Based on the results of the MRI of the brain and the stimulation tests, our case was diagnosed with growth hormone deficiency (GHD). Our case was given the green light to start the treatment of GHD with Somatropin, recombinant human growth hormone (Rastan®, Russia), at a dose of 0.594 mg/day, which was regularly adjusted (increased) according to body weight.
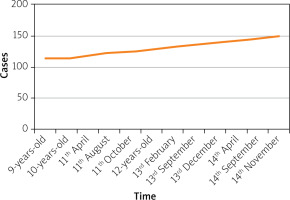
In April 2016 (11 years old), the patient for the first time started to develop some complaints of dyspeptic phenomena (abdominal pain, bloating, and violation of the stool in the form of a mushy stool). At this time, the child’s height was 120 cm, and the height gain was 7 cm in 20 months (Figure 1).
Figure 1
The flow chart of our case’s height. Upon the first examination (9-years-old), the child’s height was 113 cm (less than the third percentile height), after treatment with Somatropin, in April 2016 (11-years-old), the child’s height was 120 cm, the height gain was 7 cm in 20 months
Laboratory investigations revealed hypochromic anaemia, with a haemoglobin of 88 g/l, magnetic resonance imaging (MRI) of the pituitary was normal, and thyroid function tests showed a decrease in T4 to 0.75 ng/dl (normal range 0.78–1.34 ng/dl), which exemplified secondary hypothyroidism. To correct the level of T4, levothyroxine was prescribed at a dose of 25 µg/day. Against the background of levothyroxine intake, T4 values normalized. The doses of levothyroxine are shown in Table III.
Table III
Doses of levothyroxine
| Age | ||||||||
|---|---|---|---|---|---|---|---|---|
| 11 y | 11 y 6 m | 12 y | 13 y 3 m | 13 y 6 m | 13 y 9 m | 14 y | 14 y 6 m | |
| L-thyroxine [µg] | 25 | 33 | 50 | 62.5 | 75 | 87.5 | 100 | 100 |
Fibrogastroduodenoscopy (FGDS) showed pale and atrophied post-bulbar duodenum and the proximal part of the jejunum, and serological studies showed positive celiac-specific serology (IgA anti-tissue transglutaminase antibody and IgA endomysial antibody).
The duodenal biopsy showed grade 3 (C) subtotal atrophy of the villi, with a decreased villous-to-crypt ratio, increased cellularity of the lamina propria with a proliferation of lymphocytes and plasma cells, and an increased number of intraepithelial lymphocytes per unit length of absorptive epithelium 3–4 : 10 (normal intraepithelial lymphocyte to epithelial cell ratio, 1 : 10).
The above data allowed us to establish and justify the diagnosis: celiac disease, typical form, latent course.
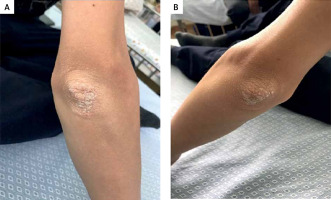
At the age of 14 years and 6 months (upon his latest follow-up visit), our case had a rare presentation among children with CD: silvery-white scales and eczema on the extensor surface of both of his elbow joints, which was diagnosed as psoriasis, and was referred to the dermatologists for treatment (Figure 2).
Figure 2
Psoriasis of the right and left elbows. Silvery-white scales on the extensor surfaces of both elbows: A – right elbow, B – left elbow
Currently, our patient is on a gluten-free diet, and his enteropathy complications ease when he stays adherent to the diet.
Discussion
We herein report a case of CD with a typical form and latent course, in a child who developed signs of growth hormone deficiency, hypopituitarism, psoriasis, and hypothyroidism. His enteropathy symptoms, which are the most common presentation of CD, did not draw the attention of his parents, but his short stature for his age and lagging behind his peers was what brought them to the outpatient clinic for help.
Association between CD and thyroid disease
The association between CD and both hyper- and hypothyroidism have been highly expressed in CD [7, 8]. Works of literature have shown that adherence to a gluten-free diet may minimize the possibility of escalating autoimmune disorders, suggesting that endocrine immunity could be due to chronic uncontrolled inflammation [9].
The pervasiveness of hypothyroidism is demonstrated in various studies to be 3 to 4 times higher risk in CD patients compared to the controls [7, 10]. A large recent study of 14,021 patients with CD found that the risk of hypothyroidism and hyperthyroidism and thyroiditis increased by 2.9 to 4.4 times compared to the general population or controls [8]. The effect of a gluten-free diet in correcting ongoing thyroid dysfunction has not yet been determined because several studies have shown conflicting results, with one study stating that firm adherence to a gluten-free diet may correct normalized subclinical hypothyroidism [10], but with other studies failing to prove so [11].
The momentum that those studies have added has favoured the recommendation of screening CD patients for thyroid function; this recommendation is also backed by both the American Association of Clinical Endocrinologists and the American Thyroid Association, especially in those with autoimmune disease [12]. It is suggested to suspect CD in patients with hypothyroidism those are not showing the expected response to levothyroxine, because the malabsorption that ensues CD might interfere with the treatment [13]. Serologic analysis of CD is recommended for patients of hypothyroidism requiring more than or equal to 125 µg/day of levothyroxine, as suggested by one study [14].
Association between CD and growth hormone deficiency
Consistently with our report, many studies have reported that in children, failure to thrive or short stature could be the initial sign of CD [15, 16]. A gluten-free diet helps most children with short stature and CD to catch up growth [16]. An Italian study reported that among children with short stature, 0.23% had both growth hormone deficiency and CD, and 0.63% had CD [17]. Concurring with the findings in our report, a gluten-free diet and growth hormone administration as combined treatment in children with growth hormone deficiency and CD can increase their growth rate [16].
Association between CD and short stature
The prevalence rate of short stature among CD patients has been an area of interest for many studies; isolated short stature cases in the absence of GIT symptoms has shown a prevalence rate between 2% and 8% [18, 19]. In a recent Saudi study of 275 children with isolated short stature reported a CD prevalence rate of 5.8% and seropositivity prevalence rate of 13.8% [20]. Short stature in CD children with GIT symptoms likewise has a high prevalence rate. A study from Egypt reported a 6.6% prevalence rate of CD among children with short stature [21]. An Indian team conducted a study on 432 children with short stature and CD with GIT symptoms, and they showed an interesting prevalence rate of 10.9% [22]. Thus, short stature unignorably presents in CD children whether GIT symptoms coexist or not.
Association between CD and hypopituitarism
Celiac disease autoimmune involvement of the pituitary gland is not adequately presented in the literature, although there are clinical and laboratory findings to support such a hypothesis. In children with CD, impairment of the growth hormone-IGF-1 axis has been frequently reported before the administration of a gluten-free diet [17], and it is generally corrected after the institution of said gluten-free diet [23]. An Italian study reported for the first time the presence of anti-pituitary antibodies (APA) in newly diagnosed children with CD, thus establishing a backbone for autoimmune involvement of the pituitary gland [24]. Another study suggested the development of autoimmune hypophysitis in children with CD who fail to catch up growth after being adherent to a gluten-free diet [25].
Association between CD and dermatologic manifestation (psoriasis)
CD patients are prone to develop skin conditions that vary from a nonspecific rash to psoriasis, eczema, atopic dermatitis, urticaria, and dermatitis herpetiform dermatitis [26].
The incidence of psoriasis among children with CD patients unlike adults is not yet expressed adequately in the literature. Several studies have discussed the benefit of a gluten-free diet on improving the severity of psoriasis in CD patients, and the explanation of such an effect of a gluten-free diet on the improvement of psoriasis could be due to the improvement of the function of the intestinal barrier or adequate absorption of vitamin D [27]. Some authors claim that psoriasis is likely to be the main clinical presentation of CD [27]. Also, typical CD antibodies such as anti-tTG, anti-endomysium IgA IgA and antigliadin IgA, IgG antibodies show a remarkable increase in psoriasis patients [28].
Conclusions
CD has a huge impact on children, which, if not promtly diagnosed and managed, might go misdiagnosed with other similar endocrinal diseases or GIT illnesses. Not all CD complications develop at the same time, and the inflammatory and/or immune response take time to develop in each case individually. Genetic causes might play a role in CD pathogenesis, and further studies are needed to investigate this hypothesis. During the course of CD, especially in children, some complications tend to frequently occur, such as growth hormone deficiency or hypothyroidism; although hypopituitarism and psoriasis are comparably rare complications of CD, they should be suspected in CD patients who exhibit atypical course. It worth mentioning that CD patients might have hyposplenism and weak response to vaccinations and higher susceptibility to infection with encapsulated bacteria [29]. With that being said, monitoring, screening, and early treatment will benefit many patients, especially children.
Evidence-based recommendations for follow-up of children with CD are lacking. Follow-up of these children should be firmly done 6 months after diagnosis and treatment initiation and then yearly, to check their symptomatic improvement, their response to treatment, and their adherence to a gluten-free diet, as well as to check if any modification of treatment is needed. Due to the unique presentation of CD in each patient, their follow-up plans should subsequently be tailored for each case.
It has been reported that children with CD rarely develop complications, and only a few cases have been reported [30]. In contrst to such reports, our case had multiple complications. Therefore, an up-to-date mindset is needed to thoroughly investigate children with CD and to be ready to deal with its various presentations and effectively manage its plethora of complications that can affect almost all the body systems.










