INTRODUCTION
Acute generalized exanthematous pustulosis (AGEP) is a rare severe cutaneous reaction. In over 90% of patients, AGEP is an adverse reaction to drugs, most frequently aminopenicillins (amoxicillin and ampicillin), quinolones, sulfonamides, chloroquine and hydroxychloroquine, terbinafine, diltiazem, ketoconazole, and fluconazole [1]. Establishing the underlying culprit drug may be very difficult initially.
Our case report describes one of the rare cases of AGEP with positive patch reaction to ceftriaxone.
CASE REPORT
Our case involves an 87-year-old female patient with a history of the right hip replacement surgery 12 years ago, arterial hypertension, dyslipidemia and heart failure, polymedicated. She had no previous history of skin conditions.
The patient was admitted to the hospital due to periprosthetic infection, for single stage revision surgery, which she went without complications. After surgery she was treated with ceftriaxone and daptomycin.
On the fifth day of antibiotics treatment the patient rapidly developed hundreds of small, sterile, non-follicular pustules on an erythematous edematous base. The rash started in the axillary and inguinal folds and spread within a few hours to the trunk, arms, and legs. The patient had no complaints of pruritus, skin pain or fever.
Laboratory blood tests showed leukocytosis (14.3 × 109/l) with neutrophilia (5.6 × 109/l) and elevated levels of C- reactive protein (75 mg/dl).
Since there was a suspicion of AGEP, the antibiotics were stopped and switched to ciprofloxacin and doxycycline.
Skin biopsy performed on the second day of the rash revealed subcorneal pustules, epidermis showing spongiosis and exocytosis of polymorphonuclear neutrophils, and papillary dermis with edema and perivascular lymphocytic inflammatory infiltrate. These morphological findings were consistent with the diagnosis of acute generalized exanthematous pustulosis.
The patient was treated with oral prednisolone 0.5 mg/kg/day and hydroxyzine for 2 weeks. The rash decreased within the following 3 days and then diminished with desquamation.
She completed 12 weeks of treatment with ciprofloxacin and doxycycline with clinical improvement.
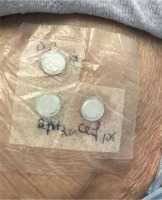
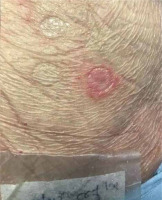
Five months after resolution of the cutaneous reaction, patch testing with ceftriaxone (10% in petrolatum [pet]) and daptomycin (500 mg 10% and 30% pet) confirmed sensitization to ceftriaxone, with the appearance of pustular lesions on an erythematous surface (Figures 1, 2). A diagnosis of AGEP induced by ceftriaxone was established. The patient was instructed to avoid all β-lactam antibiotics.
DISCUSSION
AGEP is a rare severe cutaneous rash characterized by the abrupt onset of a generalized pustular rash often accompanied by fever. Drugs, particularly antibiotics, are reported to be the major etiological factors in more than 90% of cases, but viral and bacterial infections and hypersensitivity reactions to mercury have also been associated [1, 2].
The most common associated antibiotics are not only macrolides and β-lactams but also quinolones, tetracyclines, aminoglycosides and sulphonamides. Other drugs implicated are calcium channel blockers, isoniazid, antifungals, non-steroidal anti-inflammatory drugs, paracetamol, furosemide, diltiazem, codeine, dexamethasone, carbamazepine and phenytoin [1–7].
Clinically, AGEP is characterized by the rapid development of hundreds of small, sterile, non-follicular pustules on an erythematous edematous base, starting on the main folds, with progression within a few hours to the trunk and limbs, sparing palms and soles [1]. These pustules are filled with a yellowish fluid and are often accompanied by redness and inflammation. Pustules sometimes can unite and lead to pseudo-Nikolsky evidence. Sometimes patients have fever [2].
In severe cases, there may be systemic symptoms with the involvement of internal organs, such as the liver, kidney, and lungs. However, most cases of AGEP are self-limiting and resolve within a couple of weeks once the offending medication is discontinued [1, 2].
Blood analysis usually shows leukocytosis with neutrophilia and elevated levels of C-reactive protein. One third of patients have eosinophilia [5].
The time between taking the medication and the onset of the reaction can vary, ranging from a few hours to 11 days, but it typically occurs within 48 h. Antibiotics, on average, show a median onset time of 24 h, when there is a prior sensitization to the drug [1]. In this case the patient had no evidence of a recent prior sensitization to the drug, and developed symptoms on day 5 of antibiotics treatment.
The diagnosis of AGEP is typically based on clinical presentation and a thorough medical history, including recent medication use. A skin biopsy can support the diagnosis and rule out other similar conditions [1, 2].
In this case, clinical characteristics were among the findings verifying AGEP diagnosis such as rapid spread of pustular lesions after drug onset, systemic inflammation signs, along with fast recovery following discontinuation of the suspected medications. Histopathological evidence also supported the clinical diagnosis, with the presence of subcorneal pustules, spongiosis, dermal edema and inflammatory infiltrates.
The primary treatment for AGEP involves discontinuing the causative medication immediately and supportive care. Spontaneous recovery is seen following the removal of the underlying cause [1–6]. While AGEP is not a classic histamine-mediated allergic reaction, antihistamines can still be beneficial in managing certain symptoms such as pruritus.
Using the AGEP diagnosis verification scoring formed by the Euro SCAR group, this case had a score of 12 according to this scoring system. A definitive diagnosis for AGEP has scores ranging from 8 to 12 [1].
Confirming the culprit drug is an important step in the management of cutaneous adverse drug reactions [8]. Diagnostic procedures involve controlled exposure to the causative factor and therefore carry the risk of inducing hypersensitivity symptoms [9]. Adherence to the rules of good clinical practice of patch testing along with proper training are necessary for reducing such risk [10]. Patch testing should be performed no sooner than 6 weeks after the resolution of the eruption, ideally approximately 3 months after, with the drug at various recommended concentrations and in different vehicles [1, 11, 12]. In this case, 10% concentration of parenteral ceftriaxone and 10% and 30% concentration of daptomycin in petrolatum were tested.
Patch testing positivity varies from 18% to 75%, but there are only 5 cases described in the literature of AGEP induced by ceftriaxone with positive patch tests [2–6].
β-lactam antibiotics cross-reactivity is a well-recognized concern, and individuals who are allergic to one β-lactam antibiotic may be at an increased risk of reacting to others in the same class. So, patients with a history of severe cutaneous adverse reactions associated with cephalosporin treatment should generally avoid all penicillins and cephalosporins because of the severity of the reported reaction and the not yet well-defined sensitivity of allergy tests [13].