Introduction
Tubulointerstitial nephritis (TIN) is an inflammatory process which primarily involves the renal interstitium. It is caused by toxic agents or microorganisms. The most common cause of TIN, present in as many as 70% cases, is an immunological/allergic response to non-steroidal anti-inflammatory drugs, antibiotics, and antiviral drugs. It may also develop secondarily to glomerular or renal vascular damage. Based on the duration of symptoms, TIN is categorized as acute or chronic (the latter with symptoms for ≥ 3 months). TIN usually manifests with acute kidney injury (AKI) with evidence of tubular damage. It may also be accompanied by systemic symptoms and signs including abdominal pain, vomiting, skin rash, and fever. TIN is the cause of AKI in 27% of cases in adults, and of 3 -7% of cases confirmed by biopsy in children [1-3].
Varicella zoster infection may have a very severe course in patients with nephrotic syndrome (NS), in rare cases leading even to death [4]. Kidney Disease: Improving Global Outcomes guidelines encourage vaccination of NS patients with life varicella vaccine. However, vaccination is contraindicated while on immunosuppressive or cytotoxic agents, and should be deferred until the prednisone dose is below 20 mg/day and/or immunosuppressive agents have been stopped for at least 1-3 months [5]. Also, following close contact with varicella infection, nonimmune children with NS treated with immunosuppressive agents ought to be given varicella zoster immune globulin. And once chicken pox lesions appear, the child should be started on aciclovir or valaciclovir.
We report 2 patients in whom symptoms of AKI due to TIN following aciclovir therapy were associated with recurrent NS.
Case reports
Patient 1
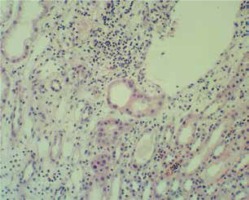
A 10-year-old boy was admitted due to a relapse of NS related to chickenpox. The patient had steroid-sensitive NS since 4 years of age and had suffered six relapses of NS. Glucocorticosteroid (GCS) therapy was discontinued 1.5 years earlier. The patient had not been vaccinated against varicella zoster virus. On admission, the patient presented with generalized edema and chickenpox rash, and laboratory tests showed nephrotic range proteinuria of 1000 mg/dl (daily urinary collection – 66 mg/kg/24 h), serum albumin level of 2.2 g/dl, serum creatinine level of 0.5 mg/dl, cholesterol level – 176 mg/dl, and triglycerides – 186 mg/dl. Therapy included aciclovir at a standard dose. In addition, the patient received twice 100 ml 20% albumin infusion, furosemide and hydrochlorothiazide. On the second day of treatment, the patient presented with abdominal pain, nausea, vomiting, and hypertension (133/82 mmHg). Laboratory tests showed evidence of AKI (Table 1), and urinalysis revealed persisting proteinuria of 800 mg/dl, with no changes in urine sediment (1 -2 leukocytes, 3 -4 erythrocytes per field of view). In immunological studies, white blood cell count was 10.0 × 103/µl, lymphocyte count 1.6 × 103/µl, neutrophil count 7.2 × 103/µl, monocyte count 1.0 × 103/µl; C3 100 mg/dl (n: 88-201), C4 19.5 mg/dl (n: 16-47), antinuclear and antineutrophil cytoplasm antibodies were negative; also negative hepatitis serology (anti-HBs 15.4 mIU/ml, negative HBs antigen and antiHCV antibodies). Ultrasound showed enlarged, hyperechogenic kidneys with reduced parenchymal perfusion. Aciclovir was discontinued. Kidney needle biopsy performed on the fourth day showed interstitial inflammatory foci with lymphocytes and single eosinophils, and heterogeneously increased cellularity and mesangial matrix within the glomeruli (Fig. 1). Acute TIN in a patient with idiopathic NS was diagnosed. Prednisone 1 mg/kg/day was initiated on the fourth day. Renal function and blood pressure normalized after 5 days of treatment, with resolution of proteinuria. Prednisone treatment was continued for 6 months.
Table 1
Laboratory test results in the studied children with nephrotic syndrome and acute kidney injury
Patient 2
An 8-year-old boy was admitted due to recurrent NS related to chickenpox. The patient had steroid-sensitive NS since 2 years of age and had suffered nine relapses of NS. He had been previously treated with prednisone, methylprednisolone pulses (8 pulses during treatment of the fifth disease relapse), cyclophosphamide, and chlorambucil. The patient had not received varicella zoster vaccination either. The patient was also diagnosed with renovascular hypertension, with computed tomography angiography showing dual arterial supply to both kidneys and a stenosis of the accessory right renal artery. On admission, the patient was treated with prednisone 1.25 mg/kg/48 hours and the antihypertensive drugs amlodipine and enalapril. Initially, the patient also presented with mild generalized edema and chickenpox rash, normal diuresis and well-controlled hypertension. Nephrotic range proteinuria of 519 mg/dl was found but without full laboratory criteria of an NS relapse (Table 1). Initial treatment included previous prednisone dose daily and intravenous aciclovir at a standard dose. On the third day of treatment, the patient presented with abdominal pain and increasing parameters of renal dysfunction (Table 1). In immunological studies, white blood cell count was 10.1 × 103/µl, lymphocyte count 3.2 × 103/µl, neutrophil count 5.7 × 103/µl, monocyte count 1.2 × 103/µl; there was normal IgM 112 mg/dl (n: 36-198), lowered IgG 269 mg/dl (n: 853-1440), normal IgA 75.4 mg/dl (n: 38-235), normal C3 121 mg/dl (n: 88-201), C4 20.1 mg/dl (n: 16-47) concentrations, antinuclear and antineutrophil cytoplasm antibodies were negative; also negative hepatitis serology (antiHBs 22.2 mIU/ml, negative HBs antigen and antiHCV antibodies), and CMV (IgM 0.08 – n: < 0.7, IgM < 4 – n: < 4 UA/ml) and EBV serology (EBV VCA IgM 0.05 – < 0.11, VCA/EA IgG 0.81 – n: < 0.09, EBNA IgG – 0.00 n: < 0.09) were found. Urinalysis showed persistent proteinuria at 300 mg/dl and 6 -10 leukocytes per field of view. Ultrasound revealed enlarged, hyperechogenic kidneys. As acute TIN related to aciclovir treatment was suspected, aciclovir was discontinued and the prednisone dose was increased to 60 mg per day. Resolution of proteinuria and normalization of renal function were seen on the sixth day of hospital stay.
Discussion
In both reported patients with NS, the primary reason for admission was a recurrence of nephrotic range proteinuria associated with chickenpox. On admission, both patients had normal renal function. Antiviral treatment with aciclovir was initiated due to an active varicella-zoster virus infection, recurrence of proteinuria, and GCS treatment (patient 1). At 2 -3 days of aciclovir treatment, both boys presented with abdominal pain and AKI.
The occurrence of AKI in children with NS is rare. In the study by Pstrusińska et al., AKI was noted in 8/1006 children (0.8%) with NS aged 6 to 17.5 years [6]. AKI may result from hypovolemia, tubular necrosis, bilateral renal vein thrombosis, acute pyelonephritis, rapidly progressive glomerular disease, and TIN [7]. A high prevalence (27%) of TIN as the cause of AKI in adults, and much lower in children (3 -7%), warrants considering this entity in the differential diagnosis of AKI [8].
Single case reports of TIN in children with active NS are available in the literature. In some cases, due to concomitant treatment with multiple medications, it is difficult to identify the agent that induced an immunologic reaction. In one case, the patient received amoxicillin, paracetamol, furosemide, and a herbal preparation [7], and another patient was treated with ciclosporin and amoxicillin-clavulanate [9]. In a third reported case, the authors associated TIN with intravenous immune globulin therapy [10].
In our patients, due to a temporal association between symptoms and administration of aciclovir, with no other causes of renal failure such as hypovolemia and thrombotic or infective complications, a possibility of a toxic effect of aciclovir was considered in the first place. Nephrotoxicity of aciclovir has been well documented [11, 12]. The drug impairs renal function by crystallization in renal tubules, particularly in patients with hypovolemia [13, 14]. In an analysis by Rao et al., glomerular filtration rate (GFR) reduction was noted in 35% (131/373) of children treated with intravenous aciclovir. Using the RIFLE score modified for the pediatric population (eGFR reduction by 25 -49% defines risk, by 50 -74% defines renal injury, and by < 75% defines renal failure), risk was found in 81 of 373 (22%) treated children, injury in 36 of 373 (9.7%) children, and renal failure in 14 of 373 (3.8%) children. This analysis showed that the risk of nephrotoxicity was higher in children treated with higher drug doses, aged > 8 years, with high body mass index, and receiving concomitant ceftriaxone treatment [15]. Aciclovir may also have a nephrotoxic effect by inducing an immunologic process that leads to development of TIN [11, 12, 16]. The disease manifests with proteinuria, renal dysfunction, leukocyturia, erythrocyturia, eosinophiluria, eosinophilia, and systemic symptoms and signs including fever, abdominal pain, rash, and arthralgia. Proteinuria is usually mild but may also be in the nephrotic range and lead to clinical and laboratory manifestations of NS [3, 17].
In our patients, AKI was superimposed on active NS. In patient 1, the diagnosis of TIN was confirmed by kidney biopsy. In addition, electron microscopy showed podocyte foot process fusion. Kidney biopsy was not performed in patient 2. In another case reported in the literature, TIN preceded NS in a child, and biopsy confirmed lesions typical for both conditions [18]. In our paper, we reported two cases of AKI due to TIN following aciclovir treatment which were superimposed on active NS. In both these patients, prompt diagnosis of TIN, discontinuation of aciclovir, and treatment with GCS led to rapid normalization of renal function and rapid remission of NS.