Introduction
Helicobacter pylori infection (H. pylori) is one of the most common human infections. According to the last 2 meta-analyses published in 2017 and 2018, the global prevalence of H. pylori infection is 44.3–45.4%, showing significant variability in different regions of the globe [1, 2].
The problem of Helicobacter pylori is relevant due to the high frequency of stomach and duodenum erosions and peptic ulcers, as well as the possible development of intestinal metaplasia and gastric cancer [3].
The Maastricht V consensus recommends an algorithm for selecting H. pylori eradication regimens based on prevalence data in a particular population of resistant H. pylori strains, because increasing resistance to H. pylori to antibiotics occurs worldwide, leading to reduced treatment efficacy [4].
Therefore, based on modern recommendations governing a differentiated approach to the appointment of a particular treatment regimen depending on the level of regional resistance to the antibiotics and local data on the effectiveness of different modes of eradication therapy [4–6], it is especially important to study the dynamics of H. pylori infection and monitor the effectiveness of eradication schemes in different regions of Ukraine.
Aim
To analyse the results of 13C-urea breath tests performed in 2006–2019, to study the dynamics of H. pylori infection and to evaluate the effectiveness of H. pylori eradication schemes in patients with the upper gastrointestinal tract (GIT) disorders – residents of the Vinnytsia region.
Material and methods
We analysed 2205 results of 13C-urea breath tests, performed during 2006–2019 in the National Pirogov Memorial Medical University, Vinnytsya clinical diagnostic gastroenterological laboratory on an infrared analyser.
In accordance with the purpose of the study, all test results were divided into 2 arrays: the first array consist of the results performed by patients with upper gastrointestinal tract disorders for the primary diagnosis of H. pylori infection – the array of primary H. pylori infection; and the second array included the results of tests performed on patients with upper gastrointestinal tract disorders to monitor the effectiveness of eradication of H. pylori – array of eradication control of H. pylori.
The results of the tests performed in patients after treatment in accordance with the recommendations of the Maastricht Consensus II, III, IV were considered by us as correct schemes. The remaining 270 (29.8%) results (140 women and 130 men who received treatment that did not meet the requirements of the Maastricht Consensus), were considered as incorrect schemes (Table I).
Table I
The main correct schemes according to the Maastricht recommendations used for eradication of H. pylori in 2006–2019
Statistical analysis
The calculation of the statistical probability of the difference between 2 samples was performed according to Student’s t-test, which was calculated for the relative values [7].
Results
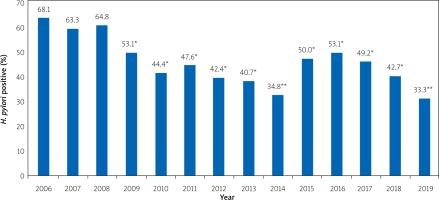
According to the results of the analysis of breath tests performed for the primary diagnosis of H. pylori infection in patients with disorders of the upper gastrointestinal tract, we found that in 2006, 2007, and 2008 the level of H. pylori infection did not differ (p > 0.05) and was 68.1%, 63.3%, and 64.8%, respectively (Figure 1). Since 2009, H. pylori infection has been significantly reduced in patients with upper gastrointestinal disorders, who have not previously received anti-helicobacterial pharmacotherapy.
Figure 1
The state of detection of H. pylori in patients with the upper gastrointestinal tract disorders (according to the analysis of breath tests with urea, performed for the initial diagnosis of H. pylori infection in 2006–2019)
*p < 0.05 compared to 2006, 2007 and 2008, **p < 0.01 compared to 2006, 2007 and 2008.
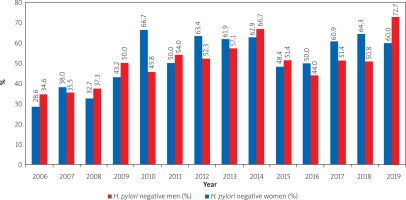
During the analysis of breath tests performed in 2006–2019 for the primary diagnosis of H. pylori infection in patients with upper gastrointestinal disorders, we did not find gender differences (p > 0.05) in H. pylori infection (Figure 2).
Figure 2
Status of primary H. pylori infection among men and women with the upper gastrointestinal tract disorders (according to the results of analysis of breath tests with urea in 2006–2019)
In a more detailed analysis of the results, we found that the lowest infection of H. pylori was observed in the age group up to 20 years – 39.6%, while in older age groups there was an increase in the percentage of H. pylori infection: 21–30 years – 46.9%; 31–40 years – 55.3%; 41–50 years – 49.5%; 51–60 years – 53.3%; and 61–70 years – 48.2%. The highest H. pylori infection rate was observed in the age group older than 71 years and amounted to 68.7%.
It should be noted that the percentage of H. pylori infected in the group under 20 years was significantly lower (p < 0.01) compared with groups 31–40, 51–60 years, and 71 and older, which was 39.6% vs. 55.3%, 53.3%, and 68.7%, respectively, while with the groups 21–30 years (46.7%), 41–50 years (49.5%), and 61–70 years (48.2%) no differences were found (p > 0.05).
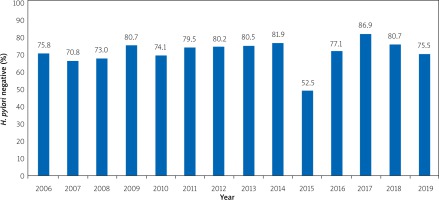
Analysing the results of breath tests with urea, which were used to monitor the success of anti-helicobacter pharmacotherapy for 2006–2019, we found that the average percentage of successful eradication of H. pylori for the entire observation period, regardless of the schemes used, was 76.4%.
It should be noted that throughout the observation period, except for 2015, the percentage of successful eradication of H. pylori was more than 70% and probably did not differ (p > 0.05) between years (Figure 3).
Figure 3
The dynamics of H. pylori eradication success from 2006 to 2019, regardless of the anti-helicobacter pharmacotherapy scheme
During further analysis of the results of C13-urea breath tests, which were performed to control the eradication of H. pylori, performed using correct schemes (according to Maastricht recommendations II, III, IV), we found that among 636 tests successful anti-helicobacter pharmacotherapy was confirmed in 517 (81.3%) cases. At the same time, among 270 results of incorrect schemes (those that did not meet the requirements of the Maastricht consensus), successful eradication of H. pylori was confirmed only in 177 (65.6%) cases.
Thus, the number of successful H. pylori eradication cases was significantly higher (p < 0.01) in the group of patients who were prescribed anti-helicobacter pharmacotherapy regimens in accordance with the Maastricht recommendations.
Given the fact that the Maastricht consensus II, III, IV revisions offer several schemes of anti-helicobacter pharmacotherapy, we conducted a comparative analysis of the main H. pylori eradication schemes success proposed in these consensuses.
We found that the successful eradication of H. pylori in the scheme proton pump inhibitor (PPI) + clarithromycin (Clar) + amoxicillin (Amox) was 81.6%, PPI + Clar + Amox + bismuth subcitrate (Bi) – 87%, PPI + Clar + metronidazole derivatives (MNZ) – 68.1%, PPI + tetracycline (TC) + MNZ + Bi – 83.9%, and PPI + Amox + levofloxacin (Lev) + Bi –76.9%.
When comparing between the studied groups (Figure 4) it was noted that the percentage of successful H. pylori eradication was significantly lower (p < 0.01) in the group PPI + Clar + MNZ (68.1%) and the group using incorrect schemes (65.6%) compared with the groups PPI + Clar + Amox (81.6%), PPI + Clar + Amox + Bi (87%), and PPI + TC + MNZ + Bi (83.9%).
Figure 4
The level of H. pylori successful eradication depending on the used anti-helicobacter pharmacotherapy schemes from 2006 to 2019
**p < 0.01 – when compared with groups of incorrect schemes and PPIs + Clar + MNZ.
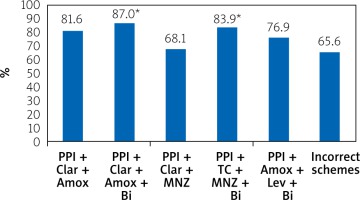
At the same time, no differences were found between the PPI + Clar + MNZ group (68.1%) and the PPI + Amox + Lev + Bi group (76.9%) and the group using incorrect schemes (65.6%) (p > 0.05).
The group PPI + Amox + Lev + Bi (76.9%) did not differ significantly (p > 0.05) from PPI + Clar + Amox (81.6%), PPI + Clar + Amox + Bi (87%), and PPI + TC + MNZ + Bi (83.9%).
Discussion
At present, the treatment of H. pylori-associated diseases is an urgent problem for the global gastroenterological community [8].
Non-invasive tests are recommended by the international community for H. pylori prevalence studies. The Maastricht consensus characterizes the 13C-urea breath test as the most studied and recommended non-invasive diagnostic method within the «test and treat» strategy [4, 9]. In our study, we analysed the results of this test.
Thus, in the analysis of the results of 1189 13C-urea breath tests, performed during 2006–2019 in the National Pirogov Memorial Medical University, Vinnytsya clinical diagnostic gastroenterological laboratory on an infrared analyser, which was performed in patients with the upper gastrointestinal tract disorders for the H. pylori infection primary diagnosis, we found that in 2006, 2007, and 2008 the H. pylori infection level did not differ (p > 0.05) and was 68.1%, 63.3%, and 64.8%, respectively.
From 2009 to 2018, in patients with upper gastrointestinal tract disorders, who had not previously received anti-helicobacter pharmacotherapy, the H. pylori infection level significantly decreased (p < 0.05).
Even more significant differences (p < 0.01) in reducing H. pylori infection were found during the urea breath test results analysis in 2014 and 2019 (34.8% and 33.3%, respectively).
Such data on the H. pylori infection prevalence are in line with the global trend (in the last two meta-analyses published in 2017 and 2018, the H. pylori infection global prevalence is 44.3–45.4% [1, 2]).
In a more detailed analysis, we found that the lowest H. pylori infection was observed in the age group up to 20 years – 39.6%, and the maximum H. pylori infection was diagnosed in the age group 71 years and older – 68.7%.
These data correlate, in particular, with the study of the prevalence of H. pylori in health care workers in Russia, which showed an increase in the proportion of those infected with age, from 41.8% in people under 25 years old to 76.9% in patients over 60 years old with a rate growth of ~ 0.7% per year [10]. In another study conducted in Novosibirsk, the prevalence of H. pylori in children aged 5–14 years was 43%, in adolescents – 55.4%, and in the age group 25–64 years – up to 70.8% [11].
Analysing the breath tests with urea results, which were used to monitor the success of anti-helicobacter pharmacotherapy for 2006–2019, we found that the average percentage of successful eradication of H. pylori for the entire observation period, regardless of the schemes used, was 76.4%.
In patients who were prescribed correct anti-helicobacter pharmacotherapy (according to the Maastricht agreements) regimens, successful H. pylori eradication was achieved in 81.3%, while in the appointment of incorrect H. pylori treatment regimens success was achieved only in 65.6% of cases (p < 0.01).
Such an important factor as the incorrect choice of eradication regimens, which, of course, affects the effectiveness of treatment, must be considered [12, 13]. Thus, the frequency of errors associated with the irrational eradication therapy use in Russia is 81% (incorrect treatment regimens, inadequate dosage, and use of drugs with unproven therapeutic efficacy) [13, 14].
According to our study, among the schemes designed according to the Maastricht recommendations, the most effective were: PPI + Clar + Amox – 81.6%, PPI + Clar + Amox + Bi – 87%, and PPI + TC + MNZ + Bi – 83.9%. The lowest efficiency was seen in the scheme PPI + Clar + MNZ –– 68.1% (p < 0.01).
It should be noted that triple therapy with clarithromycin remains the most popular eradication therapy regimen in most regions of the world. At the same time, in recent years the effectiveness of the above scheme has decreased significantly [15]. According to recent meta-analyses, the effectiveness of the triple scheme of eradication therapy is at the level of approximately 69–77% [16–18], which is slightly lower than our result.
Malfertheiner notes that the addition of bismuth to the drugs of the triple regimen allows the maintenance of high efficacy of first-line anti-helicobacter therapy, to overcome the resistance of H. pylori to clarithromycin in a particular patient, to reduce the prevalence in the population insensitive to clarithromycin, and no new strains of H. pylori antibacterial drugs with pronounced activity against H. pylori [19].
Given the above, the clinical benefits of adding bismuth tripotassium dicitrate to triple therapy drugs are being studied [20–22].
In our study, a regimen containing PPIs, clarithromycin, amoxicillin, and bismuth trical potassium dicitrate showed the highest efficiency among the regimens prescribed according to the Maastricht recommendations – 87%.
Conclusions
According to the results of the analysis of 13C-urea breath tests, performed for H. pylori primary diagnosis, it was found that in patients with upper gastrointestinal tract disorders since 2009 primary H. pylori infection was significantly reduced (p < 0.05 and < 0.01).
The lowest percentage of H. pylori infections in patients with disorders of the upper gastrointestinal tract is observed in the age group up to 20 years (39.6%), and the maximum H. pylori infection is diagnosed in the age group 71 and older (68.7%).
In 81.3% of patients who were prescribed the correct regimens of anti-helicobacter pharmacotherapy (according to the Maastricht agreements), successful H. pylori eradication was achieved, while in the appointment of incorrect treatment regimens of H. pylori success was achieved only in 65.6% of cases (p < 0.01).
Among the schemes designed according to the Maastricht recommendations, the most effective were: PPI + Clar + Amox – 81.6%, PPI + Clar + Amox + Bi – 87%, and PPI + TC + MNZ + Bi – 83.9%. The lowest efficiency was seen with the scheme PPI + Clar + MNZ – 68.1%. The difference between the PPI + Clar + MNZ group and the PPI + Clar + Amox, PPI + Clar + Amox + B and PPI + TC + MNZ + B groups was significant (p < 0.01).










