INTRODUCTION
Bronchial asthma is a heterogeneous disease characterized by chronic inflammation involving the airways, which causes airflow limitation, mucosal edema and mucus plug formation, leading to unfavorable remodeling of the bronchial wall. In the treatment regimen according to GINA, biologic therapy is proposed for patients with severe disease, used to reduce the airways inflammation, which is not controlled despite basic treatment [1]. It improves asthma control, reduces the number of exacerbations which has a positive impact on patients’ quality of life, and in most of the cases also makes it possible to reduce doses of medications including oral corticosteroids (oCS). The possibility of remission of severe asthma in the context of biologic therapy is currently under discussion, with no generally accepted definition. Remission can be achieved at different levels, such as clinical or immunological [2]. Analyses conducted to date indicate that approximately 20–40% of patients with severe asthma on therapy with biologic drugs are able to meet variously defined criteria for disease remission [3].
Asthma in the elderly has a similar prevalence to that of younger adults, however, its diagnosis and treatment can be problematic. This is primarily due to the difficulty of separating the effects of the ongoing disease process from physiological aging, a higher prevalence of comorbidities, and thus overlapping symptoms and multidirectional therapies. Elderly asthmatics have been shown to be at greater risk of severe exacerbations and death than younger patients [4], however, it appears that elderly patients treated in highly specialized centers can achieve comparable asthma control to younger patients [5]. Some of the registration studies of biologic drugs do not include the oldest patients, so little is known about the efficacy and tolerability of this type of treatment in this age group, and the available data are primarily from “real life” studies.
AIM
The aim of the study was to compare the efficacy of biological treatment and the incidence of remission of severe asthma in older and younger patients.
MATERIAL AND METHODS
The study was retrospective and consisted of an analysis of available medical records of patients with severe asthma treated with biologic therapy. The study included 75 patients who were divided into two age groups, where the cut-off was 60 years of age at the time of inclusion. Two time points were analyzed, at the 24th and 52nd week of treatment, respectively, where ventilatory parameters (FEV1% of predicted value, FEV1%pred), peripheral blood eosinophilia, response to treatment on the Global Evaluation of Treatment Effectiveness (GETE) scale and use of oral corticosteroids were measured. Asthma control was assessed by the ACQ7 (Asthma Control Questionnaire), and quality of life by the mAQLQ (mini Asthma Quality of Life Questionnaire).
INCLUSION AND EXCLUSION CRITERIAS
Patients with severe asthma whose therapy was funded by the Polish National Health Fund were eligible for the study. All patients from the center who had been treated for at least 52 weeks at the time of data collection and had two follow-up points were included in the study. Inclusion criteria for the program in brief were as follows:
EXCLUSION CRITERIA
Smoking;
Comorbidities that constitute a contraindication to therapy with a given biologic drug;
Hypersensitivity to the drug or to the excipients;
Pregnancy or lactation period.
Additional criteria for patients with allergic asthma to be treated with omalizumab or dupilumab:
The presence of allergies to perennial allergens;
Total serum IgE levels of 30–1500 IU/ml;
Establishing unequivocal in vitro reactivity to perennial allergens in patients with total serum IgE levels below 76 IU/ml.
Additional criteria for patients with eosinophilic asthma to be treated with mepolizumab, benralizumab or dupilumab:
SPIROMETRY
Spirometry was performed using a MES (Kraków, Poland) device in accordance with ERS recommendations for performing spirometry [6].
EOSINOPHILIA IN PERIPHERAL BLOOD
Cell count and differentiation were determined using an XN-1000 hematology analyzer (Sysmex, Japan).
ATOPY
Atopy was determined on the basis of the results of additional tests collected in the patients’ records. Atopy was diagnosed in the presence of at least one positive skin test or sIgE against inhalant allergens.
DEFINITION OF COMORBIDITIES
Determination of the presence of comorbidities was based on analysis of medical records or self-reporting of the disease entity by the patient.
DEFINITION OF ASTHMA REMISSION
The definition of asthma remission adopted in the study was a modification of the one proposed by Menzies-Gow et al. [7] and adapted to local conditions. Clinical asthma remission was defined as the absence of asthma exacerbations and a concurrent score of ≤ 1.5 on the ACQ questionnaire. Immunologic remission was found in patients who achieved a peripheral blood eosinophil level of less than 150/mm3 at the relevant time points. Complete remission was found in patients who simultaneously met criteria for clinical and immunologic remission and achieved FEV1 normalization (FEV1 ≥ 80% of predicted value), while complete remission without oCS was found in those who met criteria for complete remission and did not take oCS.
STATISTICAL ANALYSIS
Normality of variables distribution was assessed using Shapiro-Wilk test. In the case of normal or close to normal distribution, parametric tests were used. Categorical variables were compared using the χ2 test. Quantitative variables were presented as mean and standard deviation and were compared using Student’s t-tests for independent and dependent samples. Statistical analysis was performed using Statistica (StatSoft, Tulsa, OK, USA). P < 0.05 values were accepted as statistically significant.
RESULTS
Seventy-five subjects were included in the study, the average age was 55.4 years, 72% of the subjects were women. Atopy was present in 40 (53.3%) patients, N-ERD (NSAID-exacerbated Respiratory Disease) (based on history) was found in 18 (24%). Among comorbidities, chronic sinusitis (48%) and cardiovascular disease (44%) were the most common. Compared to the elderly, younger people were characterized by more frequent atopy and lower FEV1%pred at the time of inclusion. Of the comorbidities, only cardiovascular disease was more common in the elderly group (Table 1). The most commonly used biologic drug in the entire group was benralizumab followed by mepolizumab. Compared to older patients, younger subjects were more likely to use omalizumab.
Table 1
Demographic and clinical characteristics of patients with asthma treated with biologic drugs
Compared to older patients, younger subjects had higher ventilatory parameters at both the first and second time points (Table 2).
Table 2
Comparison of ventilatory parameters, ACQ, mAQLQ and eosinophilia in age groups at consecutive time points
CHANGE IN ASTHMA CONTROL, QUALITY OF LIFE AND SPIROMETRIC PARAMETERS (FEV1) DURING BIOLOGICAL DRUG THERAPY
During treatment, there was a significant improvement in quality of life and ventilatory parameters in the entire group at weeks 24 and 52 compared to the baseline visit; similarly, a difference was observed when assessing these parameters at weeks 24 and 52. In the case of the ACQ results, there was an improvement in asthma control at both week 24 and 52, but there was no difference between the two time points (Figure 1).
Figure 1
Change in FEV1%pred, ACQ and mAQLQ during treatment with biologic drugs in all subjects and age groups
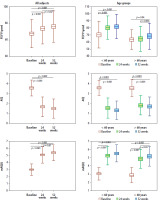
In older patients, there was a significant improvement in ACQ and mAQLQ scores at both weeks 24 and 52 compared to the baseline visit. There was no significant improvement in ACQ scores between the two time points. A statistically significant improvement in FEV1%pred occurred at week 52. There was also an improvement in this parameter when comparing weeks 24 and 52.
In younger patients, a significant improvement in ACQ, mAQLQ and FEV1%pred was observed between the baseline visit and weeks 24 and 52. There were no differences in the analyzed parameters between visits that took place in weeks 24 and 52.
ASSESSMENT OF REMISSION IN THE OLDER AND YOUNGER GROUPS
At the 24th week of treatment, 53 patients had a GETE response of good (< 60 years; 62.8%; > 60 years; 81.3%), 22 very good (< 60 years; 37.2%; > 60 years; 18.8%). Clinical remission occurred in 44% of patients, normalization of FEV1%pred in 40.3%, immune remission in 91.4%, complete remission in 23.5%, and complete remission in the absence of oCS in 17.6% (Table 3).
Table 3
Response to biological treatment in younger and older asthma patients
At the 52nd week of treatment, 48 patients had a GETE response of good (< 60 years; 61%; > 60 years; 71.9%), 25 very good (< 60 years; 39%; > 60 years; 28.1%). Clinical remission occurred in 57.5% of patients, normalization of FEV1%pred in 42.9%, immune remission in 84.3%, complete remission in 23.9%, complete remission in the absence of oCS in 22.4%.
At both the 24th and 52nd week of treatment, compared to the elderly, younger subjects showed more frequent normalization of ventilatory parameters and complete remission in the absence of oCS, and additionally, at week 52, clinical remission alone.
DISCUSSION
Asthma in the elderly can pose diagnostic and therapeutic problems, however, the treatment regimen for elderly patients remains the same as for younger adults. In the group we presented here, we confirmed that, regardless of age, therapy with biologic drugs in adult patients with severe asthma has benefits in terms of improved symptom control, disease-related quality of life and even ventilatory parameters. Similarly, there are relatively many case reports as well as real-life studies confirming not only the safety but also the efficacy of biologic drugs in elderly patients. The problem with these studies, like ours, is the usually small number of patients analyzed, and their comparison is further complicated by the fact that the age limit distinguishing younger from older patients is defined differently.
One of the first studies on this topic reported on the safety of therapy and efficacy in improving asthma control in 19 patients treated with omalizumab [8]. In another study, 109 patients with severe asthma were divided into young, middle-aged and elderly patients. Although all groups showed a reduction in the frequency of exacerbations, the need for inhaled CS and SABA, and improved FEV1 and asthma control, the extent of improvement appeared to be smallest in patients in the oldest age group [9]. On the other hand, the review paper, in which 10 studies on treatment with anti-eosinophilic drugs in the elderly were analyzed, indicated a diverse scale of improvement in parameters such as FEV1, ACT (Asthma Control Test), mAQLQ, as well as a reduction in the dose of oCS under treatment. At the same time, the authors of this study noted that only a few of them included significantly older people, although this group was not clearly defined [10]. A Spanish study of benralizumab demonstrated its efficacy and safety in elderly patients over 65 in improving control and quality of life and lung function [11]. Another study evaluated the response to treatment with benralizumab and dupilumab in older and younger patients. Both age groups showed improved asthma control, reduced frequency of exacerbations and need for prednisone, and there were no significant differences in treatment response by age. In addition, the effect of dupilumab treatment on one of the more common comorbidities associated with severe asthma, NSAID-exacerbated respiratory disease with nasal polyps (CRSwNP) was analyzed [12]. A multicenter Italian study that included 96 patients with CRSwNP showed that regardless of age, dupilumab improved symptoms, including improved sense of smell, quality of life, and objective findings on endoscopic evaluation of polyps [13]. Data from real-world evidence (RWE) studies also indicate the usefulness of biologics in the elderly population. An example is the study by Kobayashi et al. [14] which demonstrated the efficacy of mepolizumab in patients with asthma and overlapping COPD in the Japanese population.
Disease remission is now proposed as a therapeutic goal of biologic therapy in patients with severe asthma. It can be achieved at various levels including significant symptom reduction, no exacerbations, no chronic use of systemic steroids, optimization of lung function and also normalization of parameters related to T2-dependent inflammation. However, the criteria for asthma remission are not clearly defined [15]. In our group, we found that in patients over 60 years of age, the rate of complete remission in older patients not taking oCS was 4 times less frequent after 24 weeks and more than 3 times less frequent after 52 weeks of treatment, respectively, than in younger patients. This was due, among other things, to the disproportion in achieving one of the therapeutic goals, i.e. normalization of FEV1 at more than 80% predicted value, which can be explained by the progressive changes in ventilatory parameters observed with age in asthmatic patients. In addition, clinical remission was achieved in a smaller percentage of older patients. The criteria for clinical remission included the absence of exacerbations, the percentage of which was similar in both groups, and the achievement of asthma control as defined by the ACQ questionnaire of less than 1.5 points. Interestingly, at qualification, asthma control was similar; however, at both the first and second time points, older patients achieved worse, although not statistically significant, scores on the ACQ test. As a clarification, it should be noted that we used a version of the ACQ7 test, that is, one that includes FEV1. In addition, at least some studies indicate generally worse disease control in older than in younger asthmatic patients, a phenomenon that has been explained in various ways, including multimorbidity and changes due to the physiological aging process.
Our study has a number of limitations, including the retrospective nature of the study and the small patient population. It should also be noted that biologic drugs were introduced into the program in different years, and the inclusion criteria changed over time. In addition, the efficacy of biologic drugs with different mechanisms of action was analyzed simultaneously in patients with different disease phenotypes, which was related to the fact that the individual groups were too small to be compared among themselves. However, it seems that these limitations did not affect the main message of the study.
In conclusion, although clinical and complete remission were achieved less frequently in the older age group after 24 and 52 weeks of treatment, and biologic therapy was beneficial in both younger and older patients. In view of these observations, it seems that failure to meet the proposed criteria for disease remission should not influence the decision to discontinue therapy especially in the elderly.







