Purpose
Skin cancer is one of the most common types of cancer worldwide, with an ascending trend in the incidence reported every year, mainly basal cell carcinoma (BCC) and squamous cell carcinoma (SCC) [1-4]. Among several other modalities, the treatment of this type of cancer routinely involves surgery [5, 6] or radiotherapy [7]. Brachytherapy is commonly chosen in cases of skin cancer that cannot be surgically removed without serious consequences or defects of cosmetic or reconstructive procedures [8]. One of the treatment options for skin cancer is based on radiation therapy that involves placing a radiation source directly or close to a tumor while minimizing radiation exposure dose to surrounding healthy tissue [9-14]. Before starting treatment of skin cancer using radioactive source, several clinical parameters, such as total dose, fraction dose, size of target area, and total treatment time need to be optimized, as these significantly impact the final treatment result [15]. Applicator is another important parameter influencing treatment procedure. The ultimate goal is to use devices that can be tailored to obtain applicators that match patient specific skin topography. The correlation of skin topography scanning procedures, computer-assisted designing (CAD) allowing customization and fabrication based entirely on 3D printing technology, make a set of new tools in skin cancer treatment. 3D printing technology has the potential to improve the accuracy and effectiveness of skin cancer treatment by developing and creating customized applicators that precisely conform to the shape of tumor and surrounding skin [16]. A high control of tumors as well as cosmetic changes can be achieved with brachytherapy, while maintaining the highest standards of treatment [17]. In this review, we discussed the latest achievements in the field of 3D printing in skin cancer treatment, including their advantages, benefits, and limitations. We also highlighted selected studies that evaluated the use of 3D printing in skin cancer and reviewed their findings. A critical assessment of the current state-of-the-art was provided along with the indication of future directions that hopefully will stimulate the progress in the area of interest.
Material and methods
This review paper was based on a literature survey. Publications that were examined during this work were found via a dedicated scientific web browser (PubMed, Web of Science, Scopus, and Google Scholar), they were part of a scientific library of author’s publications, or were found in citation list of the reviewed publications. When gathering database, the following combination of key words were used: “HDR Brachytherapy”, “Skin Brachytherapy”, “Surface Brachytherapy”, “Contact Brachytherapy”, “3D Printing”, “Skin Cancer”, “Tumor”, “BCC”, “SCC”, “Superficial Lesions”, “Individual Applicator”, “Traditional Applicator”, “Mold”, “3D Customization”, “3D Design and Fabrication”, “Benefits of 3D Printing”, “Filaments”, “Materials”, “Radiation Source”, “Treatment Planning and Delivery”, “Clinical Report on Use of 3D Applicator”, “Biocompatibility”, “Sterilization”, and “Future Directions of 3D Printing”.
The current paper summarized selected aspects of achievements in the field of 3D printing in brachytherapy. These included the benefits of 3D printing in skin cancer treatment, advantages of printing over traditional applicators in skin cancer, traditional applicators in skin cancer, design and fabrication of customized skin applicators using 3D printing technology, evaluation of dosimetry and clinical outcomes of 3D-printed skin applicators in skin cancer treatment using high-dose-rate (HDR) brachytherapy, 3D printing technology used in brachytherapy, investigation of the material used for 3D-printed skin applicators in brachytherapy, comparison of treatment planning and delivery between traditional applicators and 3D-printed applicators in skin brachytherapy, biocompatibility and sterilization, clinical case with the use of a 3D applicator in skin cancer brachytherapy, and future directions of 3D printing in brachytherapy.
Conducting a comprehensive and systematic literature search is crucial when performing a review of 3D printing in skin brachytherapy. The process ensures that the review is based on the most up-to-date and relevant research, providing transparency and reproducibility of methodology used. In this literature search, publications specifically addressing these issues were considered. Taking into account applications of 3D printing, studies and papers that discussed various applications of 3D printing technology in the context of skin brachytherapy were identified showing how 3D printing is used to create customized applicators for the treatment of skin cancer. Regarding technical advancements, studies exploring technical aspects of 3D printing in skin brachytherapy were considered, such as advancements in printing technology and materials used. Analyzing comparative papers, studies evaluating the benefits of 3D printing in skin brachytherapy and compared with traditional methods or alternative technologies were searched. Regarding safety and dosimetry, papers on safety considerations and dosimetry in the context of 3D-printed skin brachytherapy devices were found. Challenges, limitations, and potential risks associated with the use of 3D printing technology in skin brachytherapy were examined. Also, future directions and emerging trends of 3D printing technology in skin brachytherapy as well as potential research areas and innovations were assessed. Therefore, this comprehensive approach ensured that the review encompassed full spectrum of relevant research in this field. In selecting articles for inclusion in our publication, exclusion criteria were considered to ensure quality and relevance of the chosen studies. Articles in English language related to medical description of skin cancer were included starting from 1989, and those on the appropriate topics were selected from the past few years. The most recent one was from 2023. Additionally, studies with inadequate methodology or unrelated to the research were excluded.
Benefits of 3D printing for skin cancer treatment
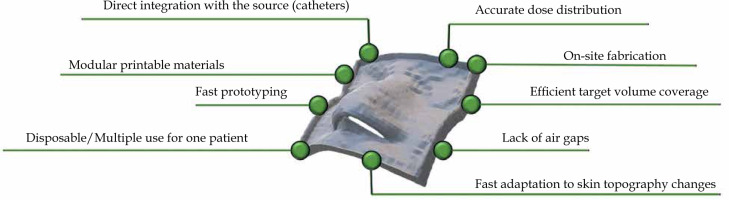
Several advantages related to 3D-printed applicators (over conventional solutions) for skin cancer treatment can be directly inferred from the current state-of-the-art. Figure 1 shows a scheme summarizing key benefits defined for the use of 3D-printed applicators. Firstly, customized applicators provide superior dose distribution in prepared treatment plans for the irradiated patient [16]. This is due to the specific placement of applicators and catheters, leading to more homogenous skin dose distribution. Also, better fitting of a 3D-printed applicator may reduce air gaps occurring between the applicator and patient skin [18]. All these influence and improve target volume coverage and organs at risk (OARs) sparing at the same time [19, 20]. Another advantage of using 3D-printed applicators is the high reproducibility of applicator position administered by 1) a single printout that can be employed several times, 2) multiple printouts that can be printed from a single g-code file and considered disposable, and 3) printouts that may be changed along with the changes of skin topography (swelling/shrinkage of the tumor, dermatological lesions) [21-23]. The overall intuitive conclusion is that 3D-printed applicators reduce variability in treatment delivery. Another undeniable benefit is the treatment adaptivity. 3D printing allows for a relatively fast creation of skin applicators [16]. In our opinion, applicators may be adjusted to changing patient needs, such as tumor swelling. These shortcomings can be solved by printing a new applicator that simply improves patient comfort as the pressure caused by the applicator is minimized. Finally, 3D printing is a cost-effective alternative, especially when compared with traditional molding techniques. It allows for the creation of high-quality applicators at a very low cost, especially when inexpensive technologies, e.g., those based on fused deposition modeling, are involved [24, 25]. One aspect that still may be considered as a challenge is related to the printing time, as the fabrication process can last for a few hours for printouts with a moderate size and shape complexity (e.g., the ear, nose). Nevertheless, the use of 3D printing in skin cancer allows personalized treatment, increase treatment efficiency in terms of dose distribution, improve treatment planning and patient comfort, and finally, reduce the cost of treatment.
Advantages of printed over traditional applicators in skin cancer
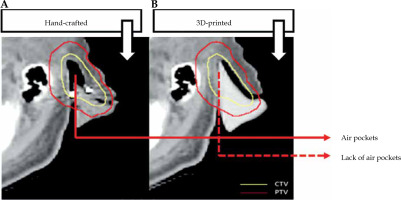
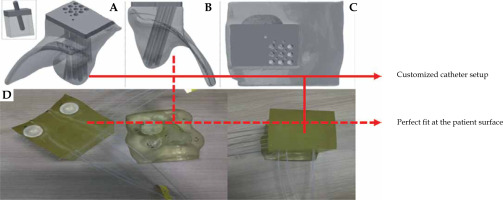
Custom applicators offer several advantages over standard ones used in skin cancer brachytherapy treatment. Computed tomography (CT) scans of patient or other surface scanning technologies [24] are used to manufacture 3D-printed applicators to perfectly fit patient surface contours. In case of CT, the region where cancer is present needs to be scanned. Based on patient anatomy, a 3D applicator can be designed and printed to fit the skin tumor and its surroundings as tightly as possible. There is no need to perform a plaster cast by a medical assistant as in case of hand-made applicators. The distance between the catheters and between the catheters and patient skin surface is another important aspect. A 3D-printed applicator can be easily adjusted to provide a larger dose to deeper located tumors, or to achieve optimal dose distribution close to an OAR while minimizing the presence of air gaps [19]. Printout engineering and placement of channels can be matched to achieve optimal dose distribution. Being challenging for hand-made applicators, it is not simple to accomplished. Reducing air gaps (as shown in Figure 2) makes a 3D-printed applicator fit exactly to patient surface (skin). Moreover, the delivery dose calculated by treatment planning system will be more accurate [26]. 3D-printed applicators can be either printed separately and further assembled, or printed by multi-material modules, resulting in hybrid materials as a mixture of catheter supports and parts acting as radiation shielding [27, 28]. Of note, printable shielding material for brachytherapy needs to be optimized and fully developed. Shielding efficacy, biocompatibility, especially with patient skin, and the correlation of shielding with absorption properties, are only a few aspects that need to be further clarified. A number of sophisticated works reported protocols allowing for the fabrication of printable materials, such as bismuth nano-particles mixed with polylactic acid (PLA) matrix [28], boron carbide polyether-ether-ketone composites [29], or a tungsten-polycarbonate polymer [30]. Such printable materials can be used as applicator parts securing OARs. Moreover, further development of different thermoplastics blended with heavy metal nano-particles [31-34] is expected to provide a set of functional materials that can be directly used in brachytherapy applications. In general, such 3D-printed shields ensure that OARs receive significantly lower irradiation doses [18, 35, 36].
Traditional applicators used for skin cancer
Traditional applicators used in skin cancer brachytherapy include surface applicators, interstitial needles, and molds [38, 39]. Surface applicators are commonly applied for superficial skin cancer treatment. These are usually made of plastic or metal, and are designed to fit patient body surface, as shown in Figure 3. The utilization of such types of applicators requires the presence of an experienced user. Different types of surface applicators can be distinguished, including Freiburg flap applicator (Figure 3A), Valencia applicator (Figure 3B), or Leipzig applicator (Figure 3C) [11, 38-46]. Leipzig and Valencia applicators are designed to treat small areas of up to 3 cm2. Interstitial needles are used for treating tumors, such as melanoma, located in deeper parts, e.g., in the skin [47, 48]. The needles are inserted directly into the tumor or tumor bed, and then secured in place.
Fig. 3
Graphical representation of three traditional applicators commonly used in skin cancer. A) Freiburg flap applicator [50]; B) Valencia applicator [50]; C) Leipzig applicator [50]

Molds are another type of applicator. These are typically made of silicone or other flexible materials, which are used to develop a customized printout of patient skin surface [10, 12, 49]. There are some restrictions in using traditional applicators, e.g., they can be unsuitable for treating tumors located in difficult to reach areas, or in patients suffering from large or irregularly shaped tumors. For each of the described applicators, there are advantages and disadvantages, and the choice of applicator depends on the size, location, and type of tumor. Overall, traditional applicators are an important component of skin cancer brachytherapy and are effective in delivering radiation to skin cancer patients. Nevertheless, we expect that 3D-printed alternatives will progressively replace conventional solutions. It is significant to note that outcomes achieved through the utilization of conventional applicators (non-3D-printed) already exhibit a high degree of excellence regarding local control, cosmetic efficacy, and toxicity profiles.
Design and fabrication of customized skin applicators using 3D printing technology
The design and fabrication of customized skin applicators using 3D printing technology is a relatively new area of research that holds great promise for improving patient outcomes in clinical applications, such as HDR brachytherapy. Researchers investigated the use of 3D printing technology to design and fabricate applicators for skin cancer or lesions located close to the skin surface [16, 20, 22, 37, 51-53]. For example, one study described the use of 3D-printed superficial applicators for skin HDR brachytherapy. The researchers used 3D-printed applicators manufactured with the use of TangoPlus FullCure930 material that was printed on a Stratasys Objet 500 Connex 1 PolyJet 3D printer. In the first step, a patient pre-planning CT scan was obtained and it was exported to treatment planning system (TPS); the ideal catheter setup was determined (as shown in Figure 4) to generate an acceptable pre-plan with proper dose distribution. To establish catheter trajectories for 3D printing, a copy of pre-plans was developed with all dwell positions activated in the catheter. Next, dwell positions were sent into a text file and imported to MATLAB software. The script creates spaces for each exported dwell position to generate channels required for the catheters, in which the source would move according to the developed treatment plan. The obtained data were exported with the original CT scan to Mimics Medical v. 18.0. This was used to develop catheter trajectories in applicator volume. The contour of patient defines the shape of applicator. The segmentation was cropped to include treatment region only and exported into 3-Matic Medical v. 10.0. Using the data sent, a box was built around the area to be treated. The box was enlarged by 80 mm to include the paths that the catheters would take and a margin to allow for a full-scatter treatment environment [54]. The MATLAB script with the trajectories planned was then imported, and catheter channels were converted into solid matter. In the next step, the box was cropped to the desired shape and size, and resulted in an applicator geometry that would correspond to patient surface with proper catheter paths. The file was exported as a .stl file and, subsequently, into g-code that was exported to the printer. The catheters were inserted into 3D-printed catheter channels after the applicator is post-processed and cleaned. The authors reported that the use of a pre-plan technique for ideal catheter placement significantly enhanced the entire process of superficial HDR brachytherapy treatment. They developed flexible, well-fitting, and high-quality applicators. Their results showed a more efficient and improved pathway for the patient [21]. Another study described a designing process for a 3D-printed patient-specific applicator for HDR brachytherapy of the orbit. The applicator was created with AutoCAD software. All data were based on a CT scan of the patient desired location. Digital imaging and communications in medicine (DICOM) structure set was exported into files using 3D Slicer [55]. Structures including the patient surface were used to develop an applicator adjusted to and filling the orbital cavity, protruding 10 mm above the surface in front of the supra-orbital ridge. Additional supporting parts were designed to provide a stable and reproducible fit to the patient surface. Channels were created to accommodate an endobronchial HDR source guide tube. The distance between the center of the channel was set to 9 mm. An acrylic photopolymer (polymerized TangoPlus® and Aqilus30® Family) was chosen as the printing material due to its human skin-imitating properties [56]. To avoid the contact with the patient skin, the applicator was wrapped in a sterile material. Agilus30® and TangoPlus® family polymers were mixed at a ratio of 20/80 for the primary applicator, whereas for the sheath, the ratio was set at 20/80. The applicator was printed on a J750 PolyJet 3D printer [51].
Fig. 4
Graphical representation of the designed and fabricated customized skin applicators. A) Model view of an orbit applicator [51]; B, C) View of the applicator at different angles [51]; D) 3D-printed applicator with the patient face printed [20]. All images from [20] were obtained with the permission of Elsevier
Overall, the design and fabrication of customized applicators using 3D printing technology represent an area of research with the potential to improve clinical applications. Tools (software and hardware) that are used to design and fabricate applicators are still under development. We anticipate that a better association between the patient CT scans, software allowing for applicator .stl file generation, and its further adaptation (e.g., via the addition of catheter cavities) will facilitate further progress in the field. While there are still some challenges that need to be addressed, the rapid pace of technological advancement suggests that the use of 3D printing technology for the design and fabrication of skin applicators will continue to grow.
Evaluation of dosimetry and clinical outcomes of 3D-printed skin applicators in skin cancer treatment using HDR brachytherapy
3D printing technology was applied to develop customized skin applicators in HDR brachytherapy, which can improve dose distribution and spare healthy tissue. The dosimetry and clinical outcomes of 3D-printed skin applicators in HDR brachytherapy were evaluated in several studies. Bassi et al. [57] analyzed the dosimetry assessment of patient–specific 3D printable materials in HDR surface brachytherapy. The objective of the study was to compare the dose determined in the presence of printed materials using iridium-192 (192Ir) gamma radiation source with the dose determined by Oncentra® treatment planning system, as shown in Figure 5. For this purpose, the researchers used GAFchromicTM (external beam therapy) EBT3 films to evaluate spatial dose measurements. The films are effective in brachytherapy dosimetry, confirmed in numerous sophisticated works [53, 58-61]. Additionally, a Physikalisch-Technische Werkstätten (PTW) microDiamond detector was installed in a PTW water phantom to assess the dosimetric properties of materials used in suitable energy and dose range [57]. Investigations were performed using three printing materials, such as NinjaFlex® (Airwolf3D), Wolfbend® (NinjaTek), and CheetahTM (NinjaTek). The measurements and analyses conducted by the researchers showed that the CheetahTM material was comparable with water at 192Ir energy. A comparison between Oncentra® Brachy and absolute dose measured at films showed a pass rate of 100% with gamma analysis, with used tolerance of 2%/1 mm distance-to-agreement (DTA). NinjaFlex® (NinjaTek) and Wolfbend® (NinjaTek) appeared to be equivalent to water, with good dosimetric representation of CheetahTM (NinjaTek) [57]. Another study presented the development and dosimetric assessment of a patient-specific elastic skin applicator in high-dose-rate brachytherapy [52]. The main purpose of this research was to create an elastic skin applicator and compare the characteristics of materials used. The materials used for printing were high-impact polystyrene (HIPS) and Dragon Skin®. Dragon Skin® received certification from the International Organization for Standardization (ISO) 10993-10 as a skin-safe material as well as ISO 10993-5, meaning that it is a clinically usable material. This product was tested and applied in a few institutes for clinical applications [62-64]. HIPS and Dragon Skin® materials have a density equal to 1.04 g/cm3 and 1.07 g/cm3, respectively. For HDR planning and exposure, the researchers used 192Ir MicroSelectron-HDR v.2 gamma radiation source, with the dose determined by Oncentra® treatment planning system. Dosimetry measurements were carried out using anthropomorphic head phantom and Gafchromic EBT3 films, which were positioned at the midline level of the desired volume and placed axially into the phantom. The source dose distribution needs to be accurately described and analyzed before measurements [65]. To validate EBT3 films and air-kerma strength, re-entrant well-type ionization chamber was used. Moreover, EBT3 films were calibrated at doses ranging from 0 to 40 Gy. Dose volumetric parameters were analyzed with a dose volume histogram (DVH). The comparison was based on three applicators, including Freiburg flap, HIPS-based, and Dragon Skin®-based materials. It was shown that minor variations presented no discernible impact on dosage distributions. While using Freiburg flap applicator, the planned DVH parameter coverage was compared with that of other applicators applied in the research. The measurements of target volume maximum doses for both HIPS and Dragon Skin® applicators were almost twice greater than those for Freiburg flap applicator. V50% for the Freiburg flap applicator was found to be larger than for the other two applicators. The maximum dose for organs was slightly lower for the HIPS and Dragon Skin® applicators. The average gamma passing rates of Dragon Skin® were higher than those of the Freiburg flap and HIPS applicators for all gamma criteria (3%/3 mm, 2%/2 mm, 1%/2 mm, 2%/1 mm, and 1%/1 mm) according to the global gamma passing rates comparing film measurements and TPS-calculated dose distribution (3%/3 mm, 2%/2 mm, 1%/2 mm, 2%/1 mm, and 1%/1 mm). The elastic applicator (Dragon Skin® material) maintained setup consistency and eliminated small air gaps, resulting in the highest gamma passing rates.
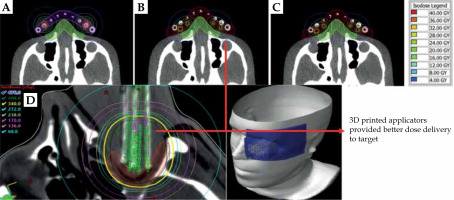
3D-printed applicators provided better dose delivery to target
Overall, flexible 3D-printed applicators demonstrated stronger adherence to the body surface. In this case, agreement between the planned and delivered dose distribution was at a high level. In addition, the flexible 3D-printed applicators provided better dose delivery to the target volume while protecting OAR compared with the Freiburg flap applicators. 3D-printed flexible applicators may be effectively implemented in HDR brachytherapy skin treatment [52].
3D printing technology used in brachytherapy
Based on the current state-of-the-art, six main 3D printing technologies were used so far to produce devices employed during brachytherapy.
Fused filament fabrication (FFF) is one of the most popular 3D printing technologies that is affordable and widely available. It produces 3D objects by melting plastic filaments via layer-by-layer extrusion. FFF printers can create reasonably big components and are typically simple to use, although the quality of final product might not be as good as with other printing techniques. Materials, such as acrylonitrile butadiene styrene (ABS; a near-water equivalent material [66]), VisiJet M2 ENT®, PLA, Ninjaflex®, and polyethylene terephthalate (PET) can be used with FFF technology [67].
Fused deposition modeling (FDM) is a 3D printing technology similar to FFF, but with higher precision and quality. To print a 3D item, molten plastic is extruded via a nozzle and deposited one layer at a time. FDM printers have become popular due to their accessibility and low cost. However, they frequently require post-processing in comparison with other printing techniques. Materials, such as PLA, polyamide 12 (PA12), ABS, and polycarbonate (PC) can be used with FDM [68].
PolyJet (PJ) is a 3D printing technology that uses photopolymerization to create high resolution parts. Layers of liquid photopolymer are jetted onto a construction tray, where they are subsequently exposed to UV curing. Complex geometries and precise details can be produced with PJ printers, which are known for their potential. Materials, such as TangoPlus® FullCure930 and Agilus 30® can be applied in PJ [69].
Multi Jet Fusion (MJF) uses a powder-based material and a liquid binding agent to produce high quality parts. It operates by carefully jetting a binding agent onto a powder bed, which is then heated to fuse the powder and form a solid item. MJF printers are well-known for their speed and capacity to create highly accurate working parts. Materials, such as PA12 can be used in MJF [70].
Stereolithography (SLA) uses a liquid photopolymer resin to produce high quality parts. It works by using layer-by-layer selective laser curing of the resin to produce a solid component. SLA printers are popular for medical applications due to their capacity to produce intricate and high resolution parts. Materials, such as Accura ClearVue can be applied in SLA [71].
Layer plastic deposition (LPD) is a type of 3D printing technology that works by extruding plastic material layer-by-layer to build a 3D object. In this process, a spool of plastic filament is fed through a heated nozzle, which melts the plastic and deposits it in precise locations to build an object layer-by-layer. Materials, such as ABS, Dragon Skin® [52, 72], and HIPS can be applied in LPD [73].
Each 3D printing technology is associated with advantages and drawbacks. FFF, FDM, and LPD are well-known for their accessibility and speed of producing larger applicators, whereas PJ, SLA, and MJF are recognized for their high resolution and accuracy. The ideal solution for a specific brachytherapy technique will rely on particular needs of the process. Additionally, we would like to encourage scientists to further explore other printing techniques that can be used in skin cancer treatment. Other techniques may bring new qualities into applicator geometry and can open access to alternative materials. Table 1 shows a comprehensive overview of the printing technologies along with materials and scanning technology applied. An indicated summary is a list of references with readily available protocols describing the design, printing, and characteristics of applicators.
Table 1
Summary of different types of brachytherapy applicators along with technical details influencing fabrication process
| Applicator type | Software | 3D printing technology | Materials used | 3D printer | Scanning technology | Ref. |
|---|---|---|---|---|---|---|
| Universal | MeshLab, MATLAB | FFF | ABS | 3D Touch | CT | [25] |
| Superficial applicator | MATLAB, Mimics Medical v.18.0 | PJ | TangoPlus FullCure 930 | Stratasys Objet 500 Connex 1 PolyJet 3D | CT | [20] |
| Skin, legs | N.A. | FFF | VisiJet M2 ENT (MJP) | ProJet MJP 2500 Plus 3D | CT | [18] |
| Facial | Beben-DICOM | FDM | PLA | Prusa i3 MK3S+ | CT | [16] |
| Facial | Mesh Mixer | FFF | PLA | BCN3D Sigma | Sense 3D Scanner | [22] |
| Universal | 3D Slicer, Solid Works 2017 | FFF, SLA | PLA | Monoprice IIIP, Creality CR-10, Form 2 SLA | CT | [24] |
| Facial + universal | Blender | MJF, FDM | PA12 | HP Jet Fusion 5200, HP Jet Fusion 580 | CT | [74] |
| Skin, universal | SketchUp, Slic3r | FFF | PLA | Fusematic 3D | N.A. | [75] |
| Right arm, phantom | Meshroom, Blender, MeshLab, Slicer 3D | LPD | ABS | Zortrax M200 3D | iPhone, CT | [76] |
| Phantom study | Blender | FFF | NinjaFlex | Lulzbot Taz 5 | CT | [36] |
| Skin tumor, arm of a human phantom | SolidWorks 2018 | FFF | PET | Prusa i3 | iPhone | [77, 78] |
| Universal | N.A. | FFF | NinjaFlex, Cerrobend | Lulzbot TAZ6 3D | CT | [78] |
| Phantom study | 3D Bolus App | N.A. | NinjaFlex, Wolfbend, CheetahTM | N.A. | N.A. | [57] |
| Skin, universal | TinkerCAD | FDM | ABS | Hamlet 3DX100 | N.A. | [61] |
| Universal | Blender, Cura | FFF | PLA | Fusematic | CT | [79] |
| Surface applicator | N.A. | FFF | ABS | UP! 3D | N.A. | [80] |
| Surface applicators | Beben-DICOM | FDM | PLA | MarkerBot Replicator+ 3D | CT | [53] |
| Penile surface | Mesh Mixer, Inventor | SLA | Accura ClearVue | N.A. | CT, dental alginate | [35] |
| Surface applicators | N.A. | N.A. | N.A. | N.A. | CT | [81] |
| Surface applicators | Mesh Mixer v.11 | FDM | PLA, NinjaFlex TPU | MakerBot Z18, LulzBot TAZ 5 | CT | [37] |
| Nose | Rhinoceros 3D & Grasshopper, Simplify3D | N.A. | PLA | N.A. | CT | [26] |
| Superficial applicator | N.A. | N.A. | Colloidal hydrogel (Cl2 and starch) | Aether 1 bioprinter | Micro-CT | [82] |
| Surface mold, nose | N.A. | N.A. | Plastic | N.A. | CT | [83] |
| Skin tumors | SolidWorks, Slic3r | SLA | Methacrylate photopolymers | Formlabs | N.A. | [84] |
| Facial | N.A. | LPD | Dragon Skin®, HIPS | Zortrax M300 | CT | [52] |
| Skin surface | G-Scan | FDM | PC | FDM TITAN TI | Optic body scan | [85] |
| Nose applicator + thermoplastic mask | 3D Slicer | N.A. | N.A. | N.A. | CT | [86] |
| Fingers | Mimics and 3-Matic | PJ | TangoPlus | Objet500 Connex1 | CT | [87] |
| Orbits | AutoCAD Inventor Suite, 3D Slicer | PJ | TangoPlus, Agilus30 | J750 PolyJet 3D | CT | [51] |
[i] ABS – acrylonitrile butadiene styrene, CT – computed tomography, FDM – fused deposition modeling, FFF – fused filament fabrication, HIPS – high-impact polystyrene, LPD – layer plastic deposition, MJF – multi jet fusion, MJP – multi jet printing, N.A. – not available, PA12 – polyamide 12, PC – polycarbonate, PET – polyethylene terephthalate, PJ – PolyJet, PLA – polylactic acid, SLA – stereolithography apparatus
Comparison of treatment planning and delivery between traditional applicators and 3D-printed applicators in skin brachytherapy
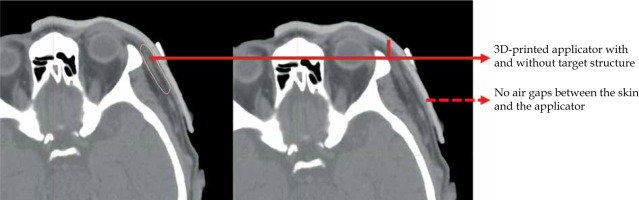
Planning the treatment of skin cancer is one of the most critical aspects, as it involves the calculation of optimal radiation dose to deliver to the irradiated target, minimizing the dose reaching surrounding healthy tissue at the same time [88-90]. The calculated dose distribution is based on the radioactive source position inside the applicator. Planning of the patient path can be challenging. The applicator shape must precisely contact the patient skin, as shown in Figure 6. Inaccurate positioning can result in sub-optimal dose distribution and possibility of under- or over-dosing [91]. Treatment planning involves the selection of an appropriate applicator that can be used in skin brachytherapy. 3D-printed applicators offer several advantages for treatment planning in skin brachytherapy. Firstly, the applicator is designed based on the patient anatomy, allowing for a custom-fit design that conforms precisely to the skin surface. This approach can improve the accuracy of dose distribution. Applicators that do not adopt to the shape of patient surface, do not allow reproducibility, which can reduce the rate of dose distribution to irradiation issues, especially due to occurrence of air gaps between the applicator and the patient skin, and algorithms used in treatment planning systems to calculate dose distribution [92, 93]. Once the 3D-printed applicator is designed, printed, and scanned with the patient, a treatment planning system is used to calculate the optimal dose distribution based on the radioactive source position. This process involves radiation source strength and activity. The treatment planning system using accurate algorithms and appropriate applicators allows for precise calculation of dose distribution to achieve the desired therapeutic effect [94, 95]. In summary, treatment planning is a critical component of skin brachytherapy, and the use of 3D-printed applicators can offer several advantages over traditional applicators, including improved dose distribution accuracy, enhanced patient comfort, irradiation of the tumor with the planned therapeutic dose, and reduced radiation exposure to healthy tissue. The precise design and calculation of dose distribution with 3D-printed applicators may contribute to better outcomes for patients undergoing skin brachytherapy.
Biocompatibility and sterilization
Biocompatibility needs to be considered when discussing medical devices applied in skin cancer brachytherapy. The term “biocompatibility” refers to the interaction between the biological tissue and biomedical devices [96, 97]. 3D printing technology offers processing methodology and biocompatible materials applied in brachytherapy. Materials used for printing applicators often have a direct contact with patient skin. In such a case, the International Organization for Standardization (ISO) is one of the organizations, which provide a set of tests for biocompatibility of medical devices [98, 99]. After passing appropriate tests and obtaining positive results, the tested material receives ISO certification. For example, materials, such as PC-ISO [100] and Dragon Skin® [52] received ISO certification, including skin safety. The use of materials without certificates is also possible, but only when a certified biocompatible coating is applied or a thick film is placed between the skin and the applicator. Eventually, other materials can be used, but only for applicators that are designed for non-medical use [25]. Generally, the materials used have to be non-toxic, non-carcinogenic, and able to withstand radiation exposures without breaking or releasing harmful substances. Sterilization is another very important aspect of applicators that have contact with patient skin. The sterilization process needs to be efficient at killing micro-organisms. However, it also has to be suitable to avoid damaging the applicator. For example, some materials may be sensitive to certain sterilization methods, while others are resistant. Ethylene oxide (ETO) sterilization method is widely applied in brachytherapy [101, 102]. ETO is a colorless, flammable gas that penetrates materials and destroys micro-organisms. It is particularly useful for sterilization of heat-sensitive materials. Heat sterilization is not recommended, as the applicator can be damaged, especially when thermoplastics, e.g., compatible with FDM or FFF, are employed. At this point, we would like to highlight that printing, in which the filament is heated to a temperature > 150°C, leads to initial sterilization. Moreover, the utilization of alcohols (e.g., ethanol or isopropanol) is not recommended when PLA, a very popular material, is used, as these solvents can affect printout structure. After each sterilization, the applicator should be inspected.
Clinical report on using 3D applicator in skin cancer brachytherapy
In a study published by Chatzikonstantinou et al. [81], clinical experience of facial skin cancer treated with the use of individualized 3D printer-based molds was explored. Fifteen patients underwent HDR brachytherapy treatment in 2020. All patients had a tumor located on the facial skin, especially in the periorbital, nasal, and cheek regions. The median age was 77 years (range, 70-90 years), and there were eight males and seven females. With regards to diagnosis, seven patients had SCC, five BCC, one patient had melanoma in situ, one lentigo maligna melanoma, and one had melanoma. The main treatment indication was microscopically non-radical (R1) resection (6 patients), radical treatment (6 patients), and recurrence after surgery (3 patients). The total prescribed dose was 39 Gy (13 fractions at daily doses of 3 Gy). Only one patient with melanoma in situ received 42 Gy (14 fractions at daily doses of 3 Gy). The median treatment time was 20 days, and the median follow-up was around 12 months. Only one patient, with melanoma, experienced a local recurrence and satellite metastasis after 16 months of follow-up. The authors also assessed acute and late toxicities. Acute radiation dermatitis grade 3 was observed in 3 patients, grade 2 occurred in 11, and grade 1 in one patient. Late toxicity as skin atrophy grade 1 was observed in 11, telangiectasia in 6, and hypopigmentation in 6 patients. One person experienced cataract grade 4. The cosmetic result was rated as good, and this was considered sufficient. Excellent local control was demonstrated in several studies [43, 103-106]. The authors concluded that brachytherapy in skin cancer performed with 3D-printed molds was a well-tolerated and safe treatment alternative for patients who were not candidates for primary surgery due to comorbidities or tumor location. With the growing prevalence of skin cancer, multidisciplinary collaboration between dermatologists and radiation oncologists is becoming increasingly important in order to provide the optimal treatment strategy for patients.
Future directions of 3D printing in brachytherapy
The analysis of the literature and our own clinical observation allowed us to conclude that technologies related to the use of 3D printing in supporting brachytherapy treatment will be developed in the coming years. The usefulness of employing applicators produced by 3D printing is supported by the already mentioned properties, such as production speed, relatively low cost, and reduction of air gaps contributing to better delivery of radiation dose to the tumor. The development of 3D printing for the need of brachytherapy in the coming years will mainly concern the issue of imaging patient body structure and faithful reproduction of geometry during printing, selection of materials for prints, and meeting expectations of clinicians and medical engineers [107, 108]. Regarding imaging of patient body structure, medical engineers have a wide range of possibilities. They can use a computed tomography (CT), magnetic resonance imaging (MRI), positron emission tomography (PET), single-photon emission tomography (SPECT), or ultrasonography (USG) [109-114]. To create a numerical model of the patient body geometry for the purpose of 3D applicator printing, medical images collected during the patient oncological diagnosis can and should be used at first. This minimizes the exposure of patient to additional doses of ionizing radiation, and allows the use of test data that may not be available in every treatment center. However, tumors may change the shape and size during cancer treatment (they are expected to shrink). In such a case, an applicator prepared on the basis of images collected before the therapy ceases to fulfill its function, and it is necessary to prepare a new applicator. This, in turn, must be preceded by collecting new images of the patient body geometry. This is where new challenges should be sought in the process of preparing applicators for HDR brachytherapy created by 3D printing. Obviously, the patient can be examined again with a tomographic method, but it exposes him to another dose of ionizing radiation. Therefore, methods of imaging that are safe for the patient as well as fast and accurate, are required. Imaging related to skin cancer is simpler because it does not require penetration into patient body; it is only necessary to map external anatomy of the tumor and its surroundings. Therefore, attempts are made to use images obtained using smartphones [77]. Such a method is fast, easily accessible, and safe for the patient. The image obtained is high resolution thanks to the quality of cameras used in mobile phones. However, it seems that this method can only be applied in mapping of flat tumors (2D) geometry. In case of convex tumors that change their shape and size during treatment process, the 2D imaging method will not work. There are high expectations from a non-invasive and safe method of laser scanning [22]. Laser devices are not expensive, and their utilization has already been documented in various fields, such as digital reconstruction of art works, reconstruction of machine parts, civil engineering applications [115], and orthopedic imaging for the purposes of rehabilitation or prosthetic limbs [116]. This method consists of illuminating the tested object with laser light in visible light range and analysis of the reflected light. Basically, such a scanner must contain a transmitter and receiver, which today’s cameras often incorporate in one device. Such a device enables non-contact measurements. Depending on the method, the measurement system analyzes reflection time proportional to the distance, phase shift of electro-magnetic wave, or deformation of raster grid illuminating the tested object [117]. As a consequence, a digitized image of the measured object is obtained, which is important from the point of view of preparing HDR applicators. Regarding skin cancers, it is possible to visualize spatial structures of such tumors. Studies showed that this method might be reproduced with an accuracy superior to 100 µm [118], which seems to be sufficient for imaging purposes for manufacturing HDR applicators, especially since accuracy of techniques, such as CT and MRI, is only an order of magnitude [119]. In terms of the selection of materials for future prints, the direction of development of materials was discussed in another part of this review paper. Basically, the development should focus on the search of materials that are inexpensive, sterilizable, and characterized by a low radiation absorption value. With regard to expectations reported by clinicians and medical engineers, it is necessary to mention the need to solve problems in the future, including processing time, accuracy, cost, and limited printing area [120]. Notably, in terms of reducing processing time, both the use of laser scanners and modern 3D printers can bring significant savings in the preparation of applicators. Similarly, accuracy is the sum of accuracy of imaging and printing methods. Both the development of imaging techniques and subsequent computer post-processing as well as the development of 3D printers will lead to an increase in the accuracy of applicators in the coming years. The reduction of production costs seems to be possible in the coming years also due to the dissemination of imaging methods (laser scanning imaging in particular) and 3D printing. Similarly, the last of the mentioned expectations of doctors and engineers, e.g., limited printing area, seems to be close to being met thanks to the development of new 3D printers, in which working area can already reach 1,000 mm × 1,000 mm × 1,000 mm.
Economic benefits of 3D printing
There are various economic benefits of 3D printing technology used in skin brachytherapy. 3D printing can significantly reduce the time and cost associated with creating custom-made molds [22]. Traditional mold-making processes require more time, which means it is a more time-consuming and more expensive process. Moreover, 3D printing has the potential to improve the efficiency and precision of treatment. Enhanced precision may contribute to achieving better patient outcomes, while the need for additional costly procedures is reduced. 3D printing has the potential to increase patient experience and satisfaction (e.g., by reducing patient discomfort). Also, the use of 3D printing can help a medical facility to achieve a competitive advantage by providing an innovative treatment option. This may attract new patients and referrals, and increase the revenue of the facility.
The overall economic benefits of 3D printing in skin brachytherapy are numerous, including reduced costs, increased efficiency, improved patients experiences, and competitive advantages of medical facilities.
Conclusions
The review showed the need for a search of modern solutions in brachytherapy of skin cancer. Modern methods, such as 3D printing of the applicator based on CT scans demonstrate significant advantages over previously used applicators prepared manually. Individual skin cancer applicators printed with 3D technology help to achieve accurate dose distribution and fewer air gaps between the applicator and patient surface. 3D-printed applicators are an alternative to traditional applicators, and are customized to fit each patient unique anatomy in skin cancer. This individualization can improve the accuracy and effectiveness of treatment, while minimizing damage to healthy tissue. When compared with traditional manufacturing methods, 3D printing technology allows for faster and more cost-effective production of applicators. In general, 3D printing of individual skin cancer applicators is a potential method of improving the precision and outcomes in radiation therapy. It is therefore advisable for radiotherapy centers to implement this type of solutions into clinical practice.