Introduction
Brain-derived neurotrophic factor (BDNF) is a member of the neurotrophin family of growth factors, which also includes nerve growth factor (NGF), neurotrophin-3 (NT-3), and neurotrophin-4/5 (NT-4/5). BDNF playing an important neuromodulatory role in the various organ systems including the brain and its associated CNS part [1–4]. In addition, BDNF is also present in several peripheral organs: the gastrointestinal tract, and the pulmonary system and their smooth muscle cells [5–7]. BDNF was initially characterized based on its crucial roles in supporting neuronal survival, differentiation, synaptic plasticity, and connectivity in the central nervous system (CNS) [1–3]. However, it is now recognized that BDNF is also highly expressed in non-neuronal tissues and exerts diverse biological effects. In particular, substantial evidence over the past 2 decades indicates the critical functions of BDNF in maintaining normal physiology, neural signalling, inflammatory responses, and epithelial homeostasis in the gastrointestinal (GI) system [4–6].
BDNF is detected throughout the gut, including the enteric nervous system (ENS), smooth muscle layers, interstitial cells of Cajal (ICCs), immune cells, and epithelia [7–10]. The expression and release of BDNF is dynamically regulated by neural, hormonal, inflammatory, and nutritional factors [11–14]. Acting through tropomyosin receptor kinase B (TrkB) receptors, BDNF signalling modulates intestinal motility patterns, epithelial transport processes, neuroimmune responses, pain pathways, and structural integrity in the GI tract [15–19].
Conversely, dysregulation of BDNF has been linked to impaired gut function and hypersensitivity in conditions such as inflammatory bowel disease (IBD), irritable bowel syndrome (IBS), and diabetic gastroenteropathy [19–22]. Restoration of intestinal BDNF levels alleviates experimental colitis, gastric motility disturbances, and visceral pain in rodent models [18, 23–25]. Hence, BDNF represents an important pathophysiological factor and therapeutic target in functional and organic GI disorders.
In this review, current knowledge on the localization, regulation, functions, and clinical relevance of BDNF in the GI system is summarized. First, the expression, synthesis, receptors, and signalling mechanisms of BDNF in the gut are outlined. This is followed by sections describing the role of BDNF in controlling intestinal motility, epithelial transport, neuroimmune interactions, visceral sensation, mucosal trophic responses, and enteric nervous system plasticity. Finally, the involvement of BDNF in the pathogenesis of IBD, IBS, functional dyspepsia, and diabetic gastroenteropathy is reviewed along with evidence that targeting BDNF may provide novel therapeutic approaches for GI diseases characterized by alterations in gut-brain interactions.
BDNF localization in the gastrointestinal tract
Early studies using reverse transcription PCR, in situ hybridization, and immunohistochemistry established that BDNF mRNA and protein are abundantly expressed throughout the GI tract. BDNF immunoreactivity has been localized to enteric neurons, interstitial cells of Cajal (ICCs), smooth muscle cells, and mucosal epithelia in mice, rats, pigs, and humans [7–9].
In particular, BDNF is highly expressed in the enteric nervous system (ENS) including neuronal cell bodies and fibres innervating the mucosa, submucosa, muscularis externa, and serosa [8, 26]. Myenteric and submucosal ganglion cells throughout the gut wall are major sources of BDNF, which supports the survival and function of enteric neurons [6]. BDNF is also synthesized by enteroendocrine cells, which secrete it basolaterally towards enteric nerve endings [27], as shown in Tables I and II.
Table I
Outlines the localization of BDNF in various cell types of the GI tract along with techniques used for the detection
Table II
Summary of BDNF localization and functions in different cell types of the gastrointestinal tract
Analysis along the rostrocaudal axis shows greater BDNF immunoreactivity in the distal colon compared to more proximal regions of rat intestine [11]. There is also a gradient along the crypt-villus axis in the small intestine with higher BDNF levels in villus epithelial cells versus crypt cells [28]. Hence, BDNF expression displays regional variations in the GI tract that may relate to its local functional roles.
Several studies indicate BDNF is present not only within the gut wall but is also released into the circulation. Plasma BDNF levels are lowered by vagotomy and elevated by intestinal inflammation, suggesting that the GI tract partially contributes to circulating pools of BDNF [29, 30]. In addition, mucosal production and basolateral secretion of BDNF by enterocytes represents a potential source of blood BDNF [27]. Intestinal epithelial BDNF may act through autocrine, paracrine, and endocrine mechanisms to maintain gut homeostasis and modulate brain-gut interactions.
BDNF synthesis and processing
Human BDNF is encoded by the BDNF gene located on chromosome 11p13 containing 11 exons [31]. Alternative splicing generates several BDNF mRNA transcript variants containing different 5’ untranslated regions. The common coding exon generates the pre-proBDNF protein comprising a signal peptide, proBDNF domain, and mature BDNF region [32], as shown in Figure 1. After cleavage of the signal peptide, proBDNF (~32 kDa) is packaged into secretory vesicles. Proteolytic processing involves cleavage of proBDNF by intracellular endoproteases, particularly furin and proconvertases, to generate mature BDNF protein (~14 kDa) [33], as shown in Figure 1.
Figure 1
Overview of BDNF synthesis, processing, and receptors in the gastrointestinal tract. The maturation and activity of BDNF is regulated through a series of processing steps and receptor interactions. BDNF is first synthesized as the precursor protein preproBDNF. This is cleaved to form proBDNF, containing both a prodomain and the mature BDNF protein sequence. ProBDNF can then be further proteolytically processed into mature BDNF (mBDNF). Both proBDNF and mBDNF are secreted, allowing them to act on cell surface receptors. The prodomain of proBDNF preferentially binds sortilin receptors, while the mature domain interacts with p75NTR to activate signalling pathways like JNK/cJUN, PI3K/AKT, and TRAF6/NF-kB that mediate cell survival and apoptosis. In contrast, mBDNF binds with high affinity to TrkB receptors, leading to activation of PLC, PI3K, MAPK, and JAK/STAT cascades. This stimulates DOWNSTREAM signalling events including CREB-mediated transcription, neuronal differentiation, and synaptic plasticity. Hence, the proteolytic processing of proBDNF to mBDNF allows a switch from apoptotic to pro-survival signalling in target neurons
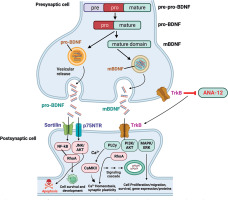
Both proBDNF and mature BDNF are biologically active and elicit distinct effects by preferentially binding to different receptors [34]. The valuation cleavage hypothesis proposes that proteolysis of proBDNF switches BDNF function from apoptotic to trophic [35]. However, the regulation of BDNF synthesis and processing is complex, and the relative levels of proBDNF versus mature BDNF affecting physiological systems remain incompletely understood.
In the GI tract, BDNF immunoreactivity is localized to both neuronal cell bodies as well as nerve terminals suggesting anterograde transport of proBDNF followed by processing and release of mature BDNF [26]. Glial cells are another source of secreted BDNF in the gut wall [36]. ProBDNF and mature BDNF differentially modulate enteric neuron survival, apoptosis, and neurite outgrowth, implicating the importance of processing [37, 38]. Further studies are required to elucidate the regulatory systems controlling BDNF protein synthesis, cleavage, secretion, and extracellular signalling in maintaining intestinal homeostasis.
BDNF receptors and downstream signalling
The biological effects of BDNF are mediated through 3 receptor systems: 1) tropomyosin-related kinase (Trk) receptor tyrosine kinases, 2) p75 neurotrophin receptor (p75NTR), and 3) Sortilin receptors [39]. BDNF preferentially binds with high affinity to TrkB, which is widely expressed in the nervous system and other tissues [40]. p75NTR is a low-affinity receptor that interacts with pro-BDNF and proneurotrophins to induce apoptosis or cell survival based on the molecular context [41]. Sortilin acts as a co-receptor with p75NTR to mediate proneurotrophin signalling [42], as shown in Figure 2.
Figure 2
Schematic flow diagram of the gastrointestinal system. Involvement of BDNF signalling in the pathophysiology of GI disorders and potential therapeutic strategies for modulating BDNF signalling. BDNF elicits its cellular effects through activation of the TrkB and p75NTR receptor systems, leading to complex signalling cascades. The full-length TrkB receptor (TrkB) is preferentially activated by binding of mature BDNF, resulting in dimerization and autophosphorylation of the receptor. This leads to the activation of downstream signalling proteins including PLCγ, ERK, and PI3K/Akt, which promote cell survival, differentiation, and plasticity. Alternatively, truncated TrkB isoforms like TrkB act through RhoA and PKC inducing cytoskeletal changes and gene regulation. On the other hand, proBDNF preferentially interacts with the low-affinity p75NTR receptor, typically alongside co-receptors like sortilin. p75NTR may either activate pro-survival TrkB pathways or pro-apoptotic JNK and Akt signalling, depending on the molecular context. Hence, both the maturation state of BDNF and the receptor complex it engages determine the downstream signalling outcomes, ranging from cell survival and neurite outgrowth to growth cone retraction or programmed cell death
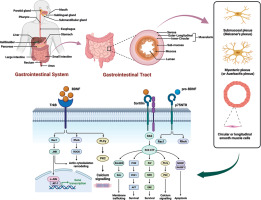
In the GI tract, activation of TrkB and p75NTR receptors stimulates distinct intracellular cascades regulating neuronal activity, epithelial functions, and immune responses. TrkB receptors are located on enteric neurons, epithelial cells, smooth muscle cells, and interstitial cells of Cajal where they mediate BDNF effects on secretion, motility, and survival pathways [16, 43]. Activation of TrkB induces receptor dimerization and autophosphorylation of tyrosine residues in the cytoplasmic domain, leading to the activation of several downstream signalling proteins [21]. These include phospholipase Cγ, phosphatidylinositol 3-kinase (PI3K)/Akt, Ras/MAPK, and phospholipase Cγ pathways regulating neuronal plasticity and cell growth [44], as shown in Figure 2.
In contrast, p75NTR receptor stimulation activates nuclear factor-κB (NF-κB), c-Jun N-terminal kinase, and RhoA signalling cascades [45]. p75NTR exhibits pro-apoptotic or pro-survival effects on neurons depending on the co-receptors involved and the molecular context [46]. The expression of TrkB versus p75NTR may determine if BDNF promotes trophic or apoptotic effects in enteric neurons exposed to inflammatory insults [47]. Elucidating the complex signalling mechanisms elicited by TrkB and p75NTR stimulation in different GI cell populations is required to decipher BDNF functions.
Role of BDNF in regulation of intestinal motility
The ENS controls intestinal motility patterns including peristalsis and segmentation critical for absorption and propulsion of luminal contents. BDNF is a key neuromodulator in the ENS that regulates neurotransmission in excitatory and inhibitory motor neurons impinging on the intestinal smooth musculature. Studies using exogenous BDNF, genetic deletion, and receptor antagonists indicate inhibitory effects on GI motility mediated by several mechanisms, as described below.
In vitro experiments showed that BDNF exposure relaxes stomach and colon smooth muscle strips through direct action on smooth muscle cells [48, 49]. BDNF induces membrane hyperpolarization and suppresses contractions in murine gastric smooth muscle by activating TrkB receptors coupled to the opening of large-conductance Ca2+-activated K+ (BK) channels [50]. The reduced Ca2+ influx due to hyperpolarization underlies the direct relaxant effect shown in Figure 2 and Table III.
Table III
BDNF regulation in the gastrointestinal tract
In vivo studies in mice lacking BDNF or TrkB receptors demonstrated increased gut motility including accelerated gastric emptying, intestinal transit, and colonic peristalsis [51–53]. Conversely, exogenous BDNF suppresses contractile activity in intestinal segments [53]. Loss of BDNF also impairs cholinergic and nitrergic neurotransmission pathways that mediate GI muscle relaxation [19, 54], as shown in Tables I and II.
The altered motility in BDNF-deficient mice may result from both direct effects on smooth muscle as well as impaired inhibitory motor neurotransmission to the muscularis externa. Additionally, BDNF modulates the excitability of enteric sensory neurons and interneurons that shape motor activity. Hence, maintaining optimal BDNF tone is required for the coordinated regulation of intestinal peristaltic reflexes.
Role of BDNF in modulation of intestinal secretion
The intestinal epithelium provides a selective barrier for the absorption of nutrients and water while blocking ingress of toxins and pathogens. Transport processes across the epithelium are tightly regulated to maintain hydration and mucosal homeostasis. BDNF signalling has been shown to enhance secretion in various intestinal epithelium models.
In cultured gastric mucosal cells, BDNF stimulates mucin release, which is important for gel-like protection of the epithelial surface [55]. BDNF also potentiates acid secretion elicited by cholinergic stimulation in parietal cells by increasing histamine release [56]. This effect is mediated by neuronal TrkB receptors as acetylcholine triggers BDNF secretion from enterochromaffin cells in gastric glands [27].
In the small intestine, BDNF augments bicarbonate transport in the duodenal mucosa, which helps protect against ulcerative damage [57]. The mechanism involves BDNF-induced phosphorylation of cystic fibrosis transmembrane conductance regulator (CFTR) resulting in increased bicarbonate fluxes.
Studies using intestinal organoids and transgenic mice showed that BDNF increases Cl- and fluid secretion in the small and large intestine by enhancing neuronal signalling [58, 59]. BDNF stimulates enteric secretomotor neuron pathways that release vasoactive intestinal peptide (VIP) and acetylcholine, which activate CFTR channels on enterocytes [60]. Thus, BDNF promotes mucosal hydration and lubrication via integrated effects on epithelial transport processes and secretory reflex circuits.
BDNF is required for maintenance of intestinal barrier integrity
A healthy intestinal epithelial barrier is crucial for preventing the entry of toxins, allergens, and microorganisms that could trigger inflammatory responses. Impaired gut barrier function with increased permeability is associated with food intolerances, inflammatory bowel disease (IBD), irritable bowel syndrome (IBS), and celiac disease [61, 62]. As a key factor regulating epithelial integrity and wound healing, BDNF deficiency may contribute to barrier dysfunction in these disorders.
Studies in cultured intestinal cells showed that BDNF enhances transepithelial electrical resistance and expression of tight junction proteins including occludin-1 and ZO-1 [63]. In vivo, mice with intestinal epithelial cell-specific deletion of BDNF exhibit reduced barrier function associated with diminished occludin and ZO-1 levels [64]. Concomitantly, these mice display exacerbated inflammation in models of colitis and food allergy [64].
BDNF strengthens the intestinal epithelial barrier through effects on tight junctions, antimicrobial proteins, and regenerative capacity [65, 66]. Following ischaemic injury in mice, BDNF stimulates enterocyte proliferation and regeneration leading to rapid mucosal restitution [67]. Impaired BDNF signalling impedes gut healing whereas exogenous BDNF restores barrier integrity after intestinal damage [68].
Overall, these findings indicate that BDNF tone is essential for maintaining the intestinal epithelial barrier and restoring its structural and functional integrity after injury. Targeting the BDNF pathway may represent a therapeutic strategy for diseases associated with increased intestinal permeability.
Immunoregulatory functions of BDNF in the gut
In addition to its neuromodulatory roles, BDNF signalling influences neuroimmune interactions and mucosal inflammatory responses in the intestine. BDNF is synthesized by intestinal epithelial cells and secreted luminally where it contributes to innate intestinal immune defences [69]. Goblet cells in the small and large intestine are also sources of secreted BDNF [70].
Studies showed that BDNF enhances IgA production by stimulating plasma cell differentiation through effects on dendritic cells [71]. Increased intestinal IgA strengthens mucosal immunity against toxins and pathogenic microorganisms. BDNF also induces the expression of antimicrobial proteins Reg3β and Reg3γ, which protect against bacterial infections [72].
However, the role of BDNF in regulating intestinal inflammation remains complex and context dependent. In vitro, BDNF protected intestinal epithelial cell lines against TNF-α and IFN-γ-induced injury [73]. The underlying mechanisms may involve inhibition of NF-κB activation and reduced expression of various proinflammatory cytokines [74], as shown in Figure 2.
Nonetheless, clinical studies showed that BDNF expression is increased in the inflamed colonic mucosa of IBD patients [75, 76]. Elevated mucosal BDNF was also reported in infectious colitis caused by Clostridium difficile [77]. Moreover, mice with intestinal epithelial cell-specific BDNF deletion exhibit reduced inflammation in chemically induced colitis [78].
These conflicting results indicate that BDNF may exert variable effects based on the disease context. While BDNF protects the epithelium against mild insults, persistent BDNF overexpression could sustain chronic inflammatory responses and damage in IBD. Further studies are required to clarify the complex role of BDNF in the regulation of neuroimmune pathways in the gut.
BDNF modulates visceral nociception and sensitivity
Visceral hypersensitivity is a key pathophysiological factor underlying chronic abdominal pain in irritable bowel syndrome (IBS) and inflammatory GI disorders. Several lines of evidence indicate that BDNF signalling enhances the excitability and sensitization of nociceptive pathways conveying visceral pain sensation.
Protease-activated receptor 2 (PAR2) stimulation, which mimics proteases released during inflammation, increases BDNF expression in colonic dorsal root ganglia (DRG) [79]. BDNF sensitizes DRG neurons by suppressing voltage-gated K+ channel currents leading to hyperexcitability [80]. Intrathecal administration of TrkB receptor antagonists reverses visceral hyperalgesia in models of stress-induced IBS [81].
In IBS patients, colonic mucosal BDNF and PAR2 expression are elevated, correlating with abdominal pain severity [82, 83]. Serum BDNF levels are also increased in diarrhoea-predominant IBS patients [84]. Stress-induced visceral hypersensitivity in rodents is associated with increased colonic BDNF levels [85].
Thus, enhanced BDNF signalling sensitizes primary afferent pain pathways and amplifies visceral nociceptive signalling from the gut to the CNS. This mechanism may underlie chronic abdominal pain in IBS and IBD. Targeting the BDNF/TrkB pathway using antimicrobials or antibodies represents a potential therapeutic approach for treating functional GI disorders [86].
Role of BDNF in the pathogenesis of inflammatory bowel disease
Inflammatory bowel disease (IBD) comprises Crohn’s disease and ulcerative colitis, which are characterized by chronic and relapsing intestinal inflammation. Altered BDNF expression and signalling has been implicated in IBD pathogenesis based on human studies and experimental colitis models [87]. However, BDNF may play complex context-dependent roles during acute injury versus chronic inflammation.
In the acute setting, BDNF levels decrease in injured colonic mucosa, which impairs epithelial restitution [88]. Exogenous BDNF administration activates cytoprotective Akt signalling and prevents apoptosis in colonic epithelial cells exposed to proinflammatory cytokines [73]. These acute protective effects were lost in cells isolated from IBD mucosa with reduced TrkB receptor expression [89].
Conversely, during established chronic IBD, BDNF expression is upregulated, which may sustain inflammatory responses [90]. Polymorphisms in the BDNF gene are associated with IBD susceptibility and severity [91, 92]. Neutralizing BDNF with TrkB-Fc fusion protein or anti-BDNF antibodies ameliorates disease in experimental colitis models [93, 94].
Hence, BDNF exhibits a dichotomous role by conferring mucosal protection acutely but enhancing chronic inflammation in IBD pathophysiology [95]. Further research is required to elucidate the complex temporal impact of BDNF signalling in intestinal inflammation before therapeutic modulation can be considered in IBD patients.
BDNF as a pathogenic factor in irritable bowel syndrome
Irritable bowel syndrome (IBS) is a chronic functional GI disorder characterized by recurrent abdominal pain associated with altered bowel habits. Altered BDNF signalling has been linked to visceral hypersensitivity and pain pathogenesis in IBS patients.
Multiple human studies show increased BDNF levels in the colonic mucosa, serum, or rectal biopsies of IBS patients, which correlate with abdominal pain severity [82, 96, 97]. BDNF immunoreactivity is enhanced in mucosal mast cells and enterochromaffin cells, which may contribute to neuronal sensitization [98, 99].
Preclinical models also demonstrate upregulated BDNF pathway activation in stress-induced visceral hypersensitivity mimicking IBS [100, 101]. TrkB antagonists administered intrathecally or intracolonically suppress visceral pain behaviours in these models [81, 102].
The mechanisms may involve both peripheral and central effects of BDNF-mediated neuronal sensitization. In the periphery, BDNF enhances afferent sensitization and neurotransmission. Centrally, increased BDNF in the spinal cord and forebrain amplifies cognitive and affective responses to visceral pain [103, 104].
Overall, enhanced BDNF signalling in key parts of the brain-gut axis contributes to pain hypersensitivity in IBS. Future studies should explore whether BDNF expression in biological samples could be a diagnostic biomarker. Targeting the BDNF/TrkB pathway with non-absorbable TrkB antibodies offers a novel approach for IBS pain management.
BDNF deficiency impairs gastric motor function in diabetes
Gastrointestinal complications frequently arise in patients with diabetes mellitus. Delayed gastric emptying and diabetic gastroparesis associated with gastric dysmotility can significantly impair glycaemic control and quality of life in diabetic patients. Animal and clinical studies indicate that BDNF deficiency may be implicated in diabetic gastrointestinal dysfunction.
Diabetic mice exhibit decreased gastric BDNF associated with pyloric sphincter hypotonia, antral hypomotility, and delayed gastric emptying [105]. BDNF infusion restores pyloric pressures and gastric motility by enhancing excitatory motor neurotransmission [106]. Diabetic gastroparetic patients also show reduced serum BDNF levels compared to diabetics with normal gastric emptying [107].
Hyperglycaemia causes BDNF deficiency in enteric neurons through effects on protein kinase C and reactive oxygen species, which impairs gastric accommodation and emptying [108]. Advanced glycation end products (AGEs), which accumulate in diabetes, also suppress BDNF and neuronal nitric oxide synthase expression, leading to depleted nitrergic inhibitory transmission [109].
Hence, the gastric motor deficits in diabetes may be partially attributed to reduced BDNF signalling. Therapies that enhance BDNF expression and release may provide benefits in the management of diabetic gastroparesis. However, clinical studies have not examined whether BDNF levels predict outcomes of prokinetic drugs or gastric electrical stimulation in diabetic patients.
BDNF regulates structural plasticity and neuroprotection in the ENS
The ENS exhibits substantial capacity for adaptive structural and functional changes during aging and GI disorders. As a key neurotrophic factor in the gut, BDNF signalling modulates neuronal plasticity, neuroprotection, and repair that maintains optimal ENS function [110], as shown in Figures 1 and 2.
BDNF enhances the survival, neurite outgrowth, and connectivity of enteric neurons [111, 112]. It protects against neuronal apoptosis induced by inflammation, oxidative stress, and neurotoxins [113]. Loss of BDNF signalling impairs the adaptive response of enteric neurons to injury [114].
In aging, BDNF expression decreases, leading to reduced neuronal density and plasticity, which may impair GI functions [115]. Enteric neurodegeneration is more severe in Alzheimer’s disease patients with comorbid BDNF gene variants [116]. Exogenous BDNF administration attenuates age-related synaptic abnormalities and neuronal loss in animal models [117].
Hence, the neurotrophic effects of BDNF counteract detrimental influences throughout life to preserve ENS structure and function. Boosting BDNF may mitigate risks of motility disorders associated with ENS aging and neurodegenerative conditions affecting the gut.
Future directions
The future research directions that could be discussed for this review on BDNF in the gastrointestinal system are as follows:
Further elucidation of the intracellular signalling cascades activated by TrkB and p75NTR stimulation in the various cell populations of the gut is needed to fully understand the mechanisms underlying BDNF’s complex biological effects.
Additional investigation into factors governing BDNF synthesis, proteolytic cleavage of proBDNF, and secretion in the intestinal mucosa could reveal new therapeutic targets for modulating BDNF tone.
Research to clarify the nuanced roles of BDNF in acute intestinal injury/inflammation versus chronic inflammatory conditions like IBD would inform when BDNF-based interventions may be beneficial or detrimental.
Development of methods for local delivery of BDNF or drugs that enhance endogenous BDNF signalling pathways specifically in the intestinal tract could provide clinical feasibility. Systemic administration risks off-target effects in the nervous system.
Analysis of BDNF protein levels in blood, intestinal biopsies, or stool samples as potential biological markers of disease severity or treatment responses should be explored for functional GI disorders.
Further study of how dysregulated BDNF signalling contributes to symptom generation in disorders like IBS and functional dyspepsia is warranted. This may lead to new therapeutic approaches.
Harnessing the neuroprotective and neuromodulatory effects of BDNF in the ENS presents a promising strategy for preventing diabetic gastropathy or improving gut function in neurodegenerative diseases.
Understanding interindividual variation in intestinal BDNF related to genetics, age, stress, microbiome, diet, and medications could help stratify patients and predict treatment responses in future clinical trials.
Further research on BDNF biology in diverse model systems and translation to patient populations will provide greater insight into its therapeutic potential for maintaining and restoring gut health.
Discussion
BDNF has emerged over the past 2 decades as a crucial factor regulating diverse aspects of gastrointestinal physiology and disease. The widespread expression of BDNF in the ENS, ICCs, smooth muscle, and intestinal epithelia enables it to modulate intestinal motility, secretion, sensation, mucosal immunity, inflammation, and plasticity through endocrine, paracrine, and autocrine mechanisms. Elucidating the complex time- and context-dependent effects of BDNF signalling in both the enteric and central nervous systems has provided greater insight into gut-brain interactions in health and disease.
Several important themes have become apparent from the accumulating studies on BDNF function in the GI tract. Firstly, BDNF signalling plays a vital role in the coordination of intestinal peristalsis and segmentation essential for normal transit. This is mediated by inhibitory effects on smooth muscle contractility and maintenance of excitatory and nitrergic inhibitory motor neuron pathways. Loss of BDNF tone disrupts the neuronal control of motility patterns. Secondly, BDNF enhances mucosal resistance and restitution after injury through stimulation of epithelial cell proliferation, tight junction protein expression, and antimicrobial defences. Deficient BDNF impairs the structural and functional integrity of the intestinal barrier.
Thirdly, the effects of BDNF on neuroimmune interactions in the gut mucosa are nuanced depending on the physiological or pathological context. BDNF exhibits acute anti-inflammatory effects but may perpetuate chronic inflammation in conditions like IBD. It also stimulates IgA production and antimicrobial protein expression, thereby bolstering mucosal immunity. Fourthly, BDNF signalling sensitizes nociceptive pathways conveying visceral pain sensations to the CNS. This mechanism probably contributes to hypersensitivity in IBS and IBD. Finally, BDNF maintains ENS homeostasis, plasticity, and repair through neuroprotective and neuromodulatory effects. Declining BDNF underlies age-related changes in gut functions.
The involvement of BDNF in key intestinal processes provides a rational basis for targeting BDNF in the treatment of GI disorders. Preclinical studies in experimental models support the therapeutic potential of BDNF modulation. For example, sequestering BDNF with TrkB-Fc fusion proteins or anti-BDNF antibodies ameliorated intestinal inflammation in colitis models, suggesting promise for IBD treatment [93, 94]. TrkB antagonists also reduced visceral hypersensitivity in stress-induced or post-infectious IBS models [81, 102].
Administering exogenous BDNF improved delayed gastric emptying in diabetic mice by restoring pyloric pressures [106]. Probiotic Bifidobacterium breve that increased colonic BDNF levels alleviated pain behaviours in a stress-induced IBS rat model [46]. Potentiating endogenous BDNF with dietary curcumin, berberine, or epicatechin ameliorated experimental colitis, diabetic gastropathy, and intestinal permeability, respectively [21, 44, 45]. Hence, both directly enhancing BDNF signalling and targeting upstream regulatory pathways may offer therapeutic approaches for functional and inflammatory GI disorders. However, some caveats need to be considered. The pleiotropic effects of BDNF imply that modulating its activity could trigger unintended consequences. Systemic administration of BDNF analogues may not be safe or practical. Local delivery methods to the intestinal lumen and ENS would be required to avoid off-target effects.
The valence of BDNF expression in acute injury versus chronic inflammation needs to be considered. For example, while blocking BDNF signalling reduced intestinal inflammation in colitis models, this may impair mucosal healing acutely after injury. The timing and duration of BDNF-based interventions requires optimization based on the disease stage. There is also large variability in intestinal BDNF levels between individuals due to genetics, age, stress, microbiota, and dietary factors [10, 14].
Determining if BDNF expression status could help stratify patients and predict treatment outcomes will be important for future clinical studies. As a secreted protein, measurement of BDNF in the systemic circulation, intestinal biopsies, or stool samples is feasible and warrants further validation as potential biomarkers [7, 29, 84]. Advancing diagnostic and therapeutic approaches based on the intestinal trophic functions of BDNF will require substantial preclinical development and validation in patient populations.
Conclusions
The neurotrophin BDNF is abundantly expressed in the ENS and intestinal mucosa where it regulates diverse physiological processes critical for GI homeostasis. BDNF signalling controls motility patterns, epithelial transport, barrier integrity, neuroimmune interactions, nociception, and ENS plasticity. Dysregulation of BDNF expression or function contributes to the pathogenesis of IBD, IBS, dyspepsia, IBD, and diabetic gastroenteropathy. Targeting the BDNF/TrkB pathway represents a promising therapeutic approach for functional and inflammatory GI disorders associated with altered gut-brain interactions. Further research on BDNF biology in cellular models, experimental disease models, and intestinal tissues from patients will provide greater insight into its complex multi-faceted roles in maintaining intestinal health.
The diverse biological actions of BDNF encompassing intestinal motility, sensation, secretion, immunity, neuroprotection, and epithelial barrier integrity underline its essential role in gut homeostasis. Dysregulation of BDNF signalling has been implicated in diseases such as IBS, IBD, functional dyspepsia, dysmotility disorders, and enteric infections. Modulating the expression or activity of BDNF represents a novel approach for GI disease treatment. However, a nuanced understanding of the dichotomous functions of this neurotrophin in acute injury versus chronic inflammation is necessary.
Elucidation of the intracellular pathways downstream of TrkB and p75NTR receptors in different gut cell populations would provide greater insight into BDNF action mechanisms. Identification of regulatory factors governing BDNF synthesis and proteolytic cleavage in the intestinal mucosa may reveal additional therapeutic targets. Development of methods for local delivery of BDNF or drugs that enhance endogenous BDNF signalling could improve clinical feasibility and minimize adverse effects.
Overall, further research on BDNF biology in the gut may enable translation of its neurotrophic functions into therapeutic strategies for maintaining intestinal health and restoring homeostasis in functional and inflammatory GI disorders.









