Introduction
Gastric cancer remains one of the leading causes of death due to cancer. Although the incidence rate of gastric cancer is continuously declining, research into the causes, risk, and prevention of gastric cancer is of high importance. The most common form of gastric cancer is adenocarcinoma, which is usually diagnosed in patients aged 45 and older [1]. It has been shown that Helicobacter pylori (H. pylori) is a recognised carcinogen that can play a causative role in the development of gastric adenocarcinoma, making the bacterium one of the causes of gastric cancer [1].
Drug consumption is considered to be a preventive, causative, or contributing factor in gastric cancer. Among the groups of frequently used drugs that have been shown to impact the development of gastric cancer are proton pump inhibitors (PPI), statins, metformin, and non-steroidal anti-inflammatory drugs (NSAID) [2]. Some cysteine derivatives, such as erdosteine and N-acetylcysteine (NAC), can be used to more successfully eradicate H. pylori and prevent or attenuate its binding to gastric mucosa [3, 4].
PPIs decrease the production of gastric acid through the inhibition of H+/K+ ATPase. They are indicated for the treatment of diseases with gastric hyperacidity, such as peptic ulcer and gastroesophageal reflux disease, to prevent NSAID-associated gastric ulcers and as a component of H. pylori eradication therapy, both triple and quadruple. The use of PPIs has been shown to be associated with an increased risk of gastric cancer [2].
Statins, or hydroxymethylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors, suppress the synthesis of cholesterol and are used for the treatment of hyperlipidaemia and to prevent the progression of atherosclerosis [2].
Metformin, a glucose-lowering biguanide derivative, is the first-line medication for the treatment of diabetes mellitus type 2. The main mechanisms of blood glucose lowering are the following: the inhibition of gluconeogenesis and glycogenolysis in the liver, the enhancement of insulin sensitivity in the peripheral muscles, and slowing the absorption of glucose in the intestines [2].
NSAIDs are a class of drugs that have anti-inflammatory effects and are thus used for pain relief and to reduce fevers. Acetyl-salicylic acid (ASA) is used in low doses as an antiplatelet medication for the primary and secondary prevention of cardiovascular events. The anti-inflammatory effect of NSAIDs occurs through the inhibition of cyclooxygenase (COX)-2 while the antiplatelet effect is mediated by COX-1 inhibition [2].
Carbocysteine, erdosteine, and NAC are derivatives of cysteine. They are used mostly as mucolytics, especially in patients suffering from chronic obstructive pulmonary disease (COPD). The mechanism of action of this class is mediated by their ability to break the intrinsic disulfide bridges that are abundant in the glycoproteins of the mucus produced by the surface goblet cells, surface secretory cells, and submucosal glands [3–5]. Worldwide, the consumption of these drugs has continuously increased, and the incidence rate of gastric cancer has gradually decreased [1, 6–12].
Aim
The aims of the current article are to evaluate the pattern and trends in the consumption of PPIs, statins, metformin, NSAIDs, and cysteine derivatives in Ukraine compared to global trends and to investigate how these drugs may influence the Ukrainian gastric cancer epidemiological indices.
Material and methods
Data on the sales of PPIs, statins, metformin, NSAIDs, and cysteine derivatives as monocomponent medications distributed through pharmacies and hospitals in Ukraine in the period 2014–2020 were collected from PharmXplorer, a market research analytical system belonging to the Proxima Research Company [13]. Daily defined doses (DDDs) per 100 000 person-years were determined for individual pharmaceutical substances and their respective pharmaceutical classes [14]. Data pertaining to the incidence of gastric cancer in the years 2014–2021 were sourced from the National Cancer Registry of Ukraine [15]. Various metrics were then derived from this dataset encompassing annual totals, age-related incidence rates, ratios reflecting different age groups for gastric cancer, and the annual incidence rates specifically for gastric adenocarcinoma.
Statistical analysis
The subsequent analysis involved the calculation of mean values, standard deviations (SD), and 95% confidence intervals (95% CI) for gastric adenocarcinoma and each age fraction/group. Trends in DDDs and the incidence rate of gastric cancer were assessed by linear regression model, and the R2-value was calculated [16]. MedCalc for Windows, version 20.218 (MedCalc Software, Ostend, Belgium), was used to compute the data.
Results
The consumption of PPIs increased gradually between 2014 and 2020 in Ukraine. The DDD rate increased by 98.61% (p < 0.0001) by 2020 compared to 2014. No drops or deviations were noted over the period (Figure 1 A).
Figure 1
Annual consumption of PPIs (A) and cysteine derivatives (B) from 2014 to 2020 (in DDDs per 100 000 inhabitants, Y-axis) in Ukraine. Note: the dotted blue and red lines are linear regression lines (equation for the blue line: y = 92867 x + 413492, R2 = 0.9595; equation for the red line: y = 4490.4 x + 46610, R2 = 0.8131)
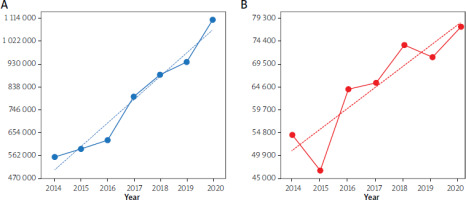
The dynamics of cysteine derivative consumption similarly increased in Ukraine in the period 2014–2020. Two drops in the DDD rate were noted: in 2015, –15.02% (p < 0.0001) compared to 2014 and in 2019, –2.44%, (p < 0.0001) compared to 2018. One leap was noted, namely, of 36.57% (p < 0.0001) in 2016 compared to 2015. A growth of 42.06% (p < 0.0001) in the DDD rate was noted in 2020 compared to 2014 (Figure 1 B).
NSAID consumption in Ukraine between 2014 and 2020 also grew. Two drops and one peak were noted in the DDD rate. The first drop was in 2015, of –9.92% (p < 0.0001) compared to 2014, and the second one was in 2020, of –3.50% (p < 0.0001) compared to 2019. The peak occurred in 2019, when there was a 26.50% (p < 0.0001) increase compared to 2015 (Figure 2 A).
Figure 2
Annual consumption of NSAIDs (A) and ASA, low doses (B) (in DDDs per 100 000 inhabitants, Y-axis) in Ukraine between 2014 and 2020. Note: the dotted yellow and orange lines are linear regression lines (equation for the yellow line: y = 43607 x + 1E+06, R2 = 0.6548; equation for the orange line: y = 6E+06 x + 1E+08, R2 = 0.6825)
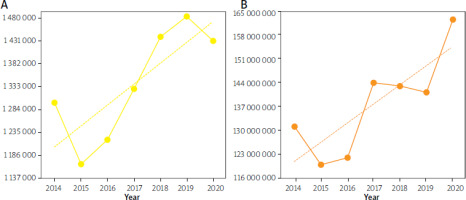
The dynamics of the consumption of ASA, in low doses, grew in Ukraine in the period 2014–2020. One drop, two slight decreases, and two leaps occurred. A drop of 8.48% (p < 0.0001) was noted in 2015 compared to 2014. Subsequent decreases were observed in 2018 and 2019 of 0.63% (p < 0.0001) and 1.30% (p < 0.0001), respectively, compared to the two preceding years. The first leap, of 17.72% (p < 0.0001) came in 2017 compared to 2016, and the second, of 14.97% (p < 0.0001), came in 2020 compared to 2019 (Figure 2 B).
The trends of the DDD rate for statins rose in Ukraine between 2014 and 2020. A 5.94% (p < 0.0001) decrease in consumption was noted in 2015 compared to 2014. There was a 2.99-fold (p < 0.0001) overall increase in consumption in 2020 compared to 2014 (Figure 3 A).
Figure 3
Annual consumption of statins (A) and metformin (B) (in DDDs per 100 000 inhabitants, Y-axis) in Ukraine between 2014 and 2020. Note: the dotted green and violet lines are linear regression lines (equation for the green line: y = 80867 x + 71949, R2 = 0.9552; equation for the violet line: y = 44385 x + 103588, R2 = 0.8924)
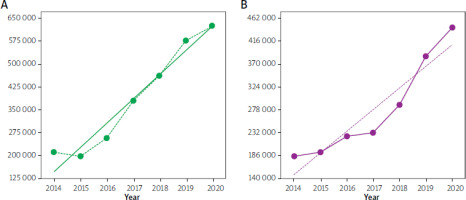
The DDD rate of metformin steadily increased, with no drops or peaks, in Ukraine during the period 2014–2020. It was 2.41-fold (p < 0.0001) greater in 2020 than in 2014 (Figure 3 B).
At the same time, the incidence rate of gastric cancer in Ukraine decreased between 2014 and 2021, having fallen by 26.56% in 2021 compared to 2014 (p < 0.0001). The most common form of gastric cancer, in Ukraine, in the period 2014–2021, was adenocarcinoma. The fraction of adenocarcinoma remained stable during the period (94.56%; 95% CI: 93.04–96.56%).
The age-related incidence rate of gastric cancer remained stable in Ukraine in the period 2014–2021. Most of the cases were diagnosed in patients aged over 45 (97.44%; 95% CI: 97.16–97.73%).
Discussion
An increasing consumption of PPIs, statins, metformin, NSAIDs, and cysteine derivatives in the period 2014–2020 and a declining gastric cancer incidence rate in the period 2014–2021 in Ukraine were noted. Gastric adenocarcinoma remained the most common form of gastric cancer, and the age of diagnosis was over 45 years. These trends correspond to those seen globally [1, 6–12].
As regards the increased consumption of PPIs, some studies suggested that the use of PPIs was associated with a heightened risk of gastric cancer, but no causative role could be confirmed due to the heterogenicity of the data [2, 17]. Moreover, a study of H. pylori adhesion showed that acid suppression may facilitate blood group binding adhesin (BabA)-mediated binding of the bacteria (BabA binds to Lewisb antigen expressed on the surface of gastric mucosa) [18]. Interestingly, the increasing consumption of PPIs did not lead to an increased gastric cancer incidence rate or meaningful shifts in the nosological structure of the morbidity and age-related incidence rates. One of the most recent meta-analyses showed no significant association between the use of PPIs and an increased risk of gastric cancer [19].
The discrepancies between the increased use of PPIs and increased rate of gastric cancer can be explained as follows. First, some PPIs can be used as part of H. pylori eradication therapy, but these data are lacking; thus, this question can only be answered by the analysis of patient-linked data on the therapy and its outcomes [2]. Second, some drugs, such as cysteine derivatives, can inhibit BabA-mediated adhesion of H. pylori and consequently improve the outcomes of eradication therapy and prevent the colonisation of gastric mucosa [3, 4]. In a clinical trial, the addition of erdosteine significantly increased the rate of successful eradications [4]. The inhibition of the binding was described for NAC and was mediated by its ability to reduce cysteine loop 2, which is crucial for the stability of the adhesin’s structure and affinity to its ligand [3]. Third, the consumption of cysteine derivatives increased.
The increased consumption of statins might play a role in preventing gastric cancer. This prevention is associated with the pharmacological mechanism of action of this class of drugs [2, 20]. The precise biological mechanisms underlying the risk reduction of gastric cancer through statin use remain uncertain. Nevertheless, several biological pathways may offer insights into their association. By inhibiting HMG-CoA reductase in the mevalonate pathway, statins inhibit the synthesis of cholesterol, dolichol, and coenzyme Q10 and downregulate the activation of the signalling pathways involved in tumour propagation and progression. Previous research has also indicated the antitumour activity of statins, such as their ability to enhance apoptosis and inhibit angiogenesis, both of which are downstream effects of the mevalonate pathway [20].
The growing use of metformin could also be associated with a lower risk of gastric cancer. The anticancer mechanism of action of metformin is associated with its ability to activate adenosine monophosphate-activated protein kinase (AMPK) and suppress the insulin-like growth factor (IGF)-1 and epithelial growth factor (EGFR) pathways. The activation of AMPK counteracts tumour progression and propagation. The inhibition of the IGF-1 and EGFR pathways leads to the prevention of tumour initiation and proliferation [2, 21].
The increased consumption of NSAIDs is known to play a role in the prevention of cancer. Their mechanism of anticancer action is mostly mediated by their ability to inhibit the enzyme cyclooxygenase (COX)-2 and thus prostaglandin (PG) synthesis, primarily PG E2. The upregulation of COX-2 is associated with angiogenesis, proliferation, inflammation, antiapoptotic pathways, and tumour invasion. PG E2 has been shown to be associated with tumorigenesis and cell proliferation. An indirect mechanism has also been described for NSAIDs and it is linked to the accumulation of arachidonic acid and activation of apoptosis [2, 22].
The results suggest that statins, metformin, NSAIDs, and cysteine derivatives play a role in preventing gastric cancer whereas PPIs may play an attributive role in the disease. The role of the drugs should be studied more carefully even though experimental results support their effect [2–4, 17, 18, 20–22]. To better study the impact of these commonly used drugs on the risk of gastric cancer, individually linked data are required on gastric cancer genetic predisposition, H. pylori infection status, eradication attempts and their success, use of drugs (both prescription and over-the-counter ones), and lifestyle [2, 23]. The digitalisation of the healthcare system and creation of data storage facilities would facilitate the extraction and analysis of the data [24].
Conclusions
The consumption of PPIs (+98.61%), statins (+199.15%), metformin (+141.29%), NSAIDs (+23.51%) and cysteine derivatives (+42.06%) increased in Ukraine from 2014 to 2020. These trends of the consumption were similar to those observed worldwide for the respective group of drugs.
The total gastric cancer (–26.56%) and gastric adenocarcinoma incidence rate decreased in Ukraine in the years 2014–2021. Adenocarcinoma remained the most common form of gastric cancer (93.04–96.56%). Most of the cases were diagnosed in the patients older than 45 years (97.16–-97.73%). These trends in the gastric cancer epidemiological indices in Ukraine resembled the global trends for the disease.
The use of statins, metformin, and NSAIDs can prevent gastric cancer through their unspecific action against tumorigenesis, cancer progression, and propagation whereas cysteine derivatives might improve the H. pylori eradication therapy outcome rate and alleviate the colonisation ability of the bacteria.
To confirm the contributing role of PPIs and the preventive role of statins, metformin, NSAIDs, and cysteine derivatives in the development of gastric cancer, further studies are required. Individual-linked data on gastric cancer predisposition, H. pylori-infection status, the success rate of H. pylori eradication therapy, the use of PPIs, statins, metformin, NSAIDs, and cysteine derivatives, and lifestyle would be of great assistance in solving the task. Such data could be made available through the further digitalisation of the healthcare and drug supply systems in Ukraine.










