INTRODUCTION
The cardinal motor symptoms of Parkinson’s disease (PD) include Parkinsonian resting tremor, rigidity and bradykinesia [1]. Dopaminergic medications encompassing levodopa and dopaminergic agonists are the gold standards in the treatment of PD but can be insufficient in patients with advanced stages of this neurodegenerative disorder [2]. Most patients treated in the long-term with dopaminergic medications develop serious side effects, mainly severe levodopa-induced dyskinesia [3].
To improve motor functions and the quality of life, especially in patients suffering from severe dyskinesia when on medication and severe motor symptoms when off medication, neurosurgical methods can be applied. Deep brain stimulation (DBS) of the subthalamic nucleus (STN) has already proved to be a reliable and effective therapy with many years of clinical usage and evidence of efficacy behind it [4]. The DBS system is composed of stereotactically implanted DBS leads, connectors and implantable pulse generators (IPGs) placed in most cases in the subclavicular area. There are two distinct types of IPGs: the non-rechargeable and the rechargeable. Non- rechargeable IPGs require replacement when depleted. The most common complications after IPG replacement are erosion and infection [5]. These complications require antibiotics, wound debridement, or removal of the DBS system in case of widespread infection.
DBS system components can be damaged in many different ways that can lead to the necessity of their removal. The most common cause for a mechanical malfunction of DBS hardware is the direct injury to the implanted DBS system elements [6]. As stated above, non-rechargeable IPGs require replacement after a period of time due to their depletion [7]. IPGs are usually replaced under local anesthetics.
Patients with long-standing PD may develop psychiatric side effects due to their long-term intake of levodopa; they may develop visual and phonic hallucinations but also obsessive-compulsive behaviors. The prevalence of psychotic complications is significantly higher than in healthy people [8]. In addition to this, symptoms like confusion, hallucinations, and delusions can develop at a much younger age in patients suffering from PD [9]. Patients with obsessive-compulsive disorder are also more likely to develop PD during their life span [10, 11].
To the best of our knowledge, this is the first report of probably self-injurious behavior after a standard IPG replacement. Patient’s behavior resulted in the damage of the entire connector, with the displacement of the left electrode leading to the disruption of the left-sided DBS. The patient self-removed the part of the connector mentioned, which resulted in skin erosion and subsequent infection. The consequence was the removal of the left-sided DBS system, with concomitant neurological deterioration.
CASE DESCRIPTION
We present the case of a 70-year-old woman with a 22-year history of PD. The primary therapy for her condition was levodopa (a daily dose of 1800 mg) and benserazide. She had severe levodopa-induced dyskinesia, which was more pronounced in the left hemibody, and unpredictable on-off fluctuations. The formal neuropsychological assessment before surgery was normal. The Mini-Mental State Examination (MMSE) before the bilateral staged STN DBS scored 28 points. The levodopa test was positive. The mean Unified Parkinson’s Disease Rating Scale (UPDRS) before surgery in medication off condition was 54, and in medication on condition it was 22 points. Magnetic resonance imaging showed no structural changes. Due to the insufficiency of the pharmacotherapy she was referred to the neurosurgery department for surgical treatment. At age 59, she underwent a staged bilateral implantation of DBS leads to both STN. Due to more pronounced Parkinsonian motor signs in the left extremities the first electrode was implanted in the right STN and after 8 months in the left STN. The patient was implanted with Medtronic DBS hardware, with both single-channel non-rechargeable implantable pulse generators (Activa 37603, Medtronic, Minneapolis, United States). The surgeries were uneventful, and the patient gained significant improvement in her motor symptoms and an almost total amelioration of the levodopa-induced dyskinesia. The patient did not suffer from compulsive behaviors and was not diagnosed by the treating neurologist, over the entire duration of her PD, with foreign hand syndrome. Over the course of her illness, the non-rechargeable IPGs became nearly totally depleted and were replaced at 57 months on the right side, and at 50 months on the left side after initial DBS STN surgeries. Both replacements were uneventful, and the patient again displayed clinical improvement. She had scheduled follow-up visits at our out-patient clinic, and the IPGs were again replaced before the total depletion. The right IPG was replaced after 54 months and the left after 55 months. All motor PD symptoms after the replacements were properly managed, in off medication and on medication condition. At each scheduled replacement of the IPGs the impedance of the implanted DBS hardware was always checked. During the last visit to the neurosurgical department for the planned replacement of the left IPG the patient’s daily levodopa dose was Madopar 125 mg taken seven times a day and one Madopar HBS 125 taken before sleep. This medication regime was stable and was not changed over the last years of the duration of the patient’s PD. For comparison, before the staged bilateral STN DBS the daily levodopa dose was nearly doubled and consisted of Madopar 250 mg, also taken seven times a day. The bilateral STN DBS contributed to a nearly 50% decrease in the daily levodopa dose over the entire follow-up period.
During the last left-sided IPG replacement the therapeutic impedances measured intraopetarively were 671 Ohm, 6.2 mA. During the patient’s last hospitalization, the impendences were also checked on the right side. The measured values were 494 Ohm, and 5.2 mA. These values were also normal. The impendences of the individual contacts of both implanted leads were normal. The patient’s MMSE during hospitalization was 26 points reflect- ing cognitive impairment without dementia. As stated above, the patient was implanted with standard DBS lead type 3389, constructed especially for the STN DBS. The patient’s final stimulation settings on the right side were 2.6 Volts, 60 microseconds, and 80 Hertz. The stimulation settings on the left side were as follows: 4.2 Volts, 60 microseconds, and 80 Hertz. On both sides, we used the bilateral monopolar stimulation mode, choosing two middle contacts on the left side (contacts numbered 1 and 2) and two contacts (numbered 1 and 3) on the right side. All active contacts were set as cathodes, and IPGs as anodes. The last left-IPG replacement surgery was uneventful. After the initiation of the left STN DBS the Parkinsonian motor symptoms in the patient’s off medication condition were greatly improved. The right-sided levodopa-induced dyskinesia also disappeared under the reemergence of the left STN DBS. The patient was given standard care for wound dressing in the area of the newly implanted IPG in the left subcalvicular area. On the day of discharge there was no subcutaneous hematoma or seroma in the area of the newly implanted IPG. The wound healed properly. The patient was taken home by her family members once they were instructed again on how to care for the wound. The patient’s son in particular already had past experience of changing the dressing over the depleted and replaced IPGs. The patient was discharged on the 5th day after the operation; she had gained clinical benefit and walked without assistance at the time of her discharge from the neurosurgical department. Her stitches were removed as usual by a local hometown surgeon. The patient did not attend her appointment for the inspection of the wound one month later, which was scheduled to take place at our neurosurgical out-patient clinic.
Two months after the left IPG replacement, in May 2022, the patient was admitted to the neurosurgical department on an emergency basis, because of self-inflicted damage to the connector and the extracranial part of the left electrode (Figure 1A). She had been abrading the skin in the left parietal area, where the connector was implanted subcutaneously. The connector and the part of the connection wire were brought were brought by family members (Figure 1A). The parts of the connector protruding from the parietal wound over the implanted connector site were clearly visible (Figure 1B). At admission, the connector and part of the electrode had ripped through the margins of the wounds, severing the link between the electrode and an IPG, and effectively switching off the stimulation. The patient presented right- sided extrapyramidal symptoms due to the malfunctioning of the left DBS system – mainly severe tremor in the right upper extremity with rigidity and bradykinesia. The son of the patient declared that his mother had not complained of excessive itching of the wound. At an urgent re-administration the MMSE was 24 points, worse than during the previous hospitalization for standard exchange of the depleted left IPG.
Figure I
A) The part of connector removed by patient and brought by her family to the neurosurgical department. B) The part of connector protruding from parietal wound over the removed connector by the patient. C) The frontal wound in electrode implantation area with visible scar from operation and sings of infection at the connector side as well as in the frontal area. D) Anterior X-ray showing the damage to the left connector (arrows). E) Computed tomography CT scan with arrows indicating the two ends of the torn out connector. F) The sagittal CT scan image with arrow showing the place on the neck with torn connector and displaced intracranial electrode
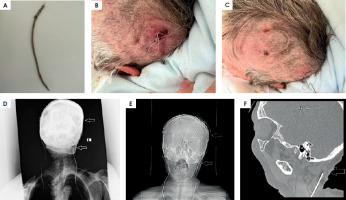
Repetitive and chronic stereotypical acts but also possible scratching of the wound resulted in this case in direct physical damage of the reimplanted DBS system. As a consequence of this behavior skin erosion and subsequently localized skin infection with signs of inflammation developed. This inflammation may have resulted in intensified itchiness and the patient’s scratching. Also, the skin in the left frontal area had signs of inflammation, suggesting that the infection had spread not only to the connector side, but to other components of the left DBS system (Figure 1C). Before surgery, X-rays of the head and neck as well upper chest were done, revealing the damage done to the left connector (Figures 1D and 1E). Computed tomography (CT) was also done to exclude the possible displacement of the left intracranial DBS electrode. The head CT also excluded the possibility of intracranial infection, and showed the disconnection of the left connector, i.e., the absence of the connector and distal extracranial part of the electrode (Figure 1F).
Treatment with intravenous antibiotics was initiated immediately. The patient underwent urgent surgery, during which the whole left-sided DBS system was removed, including the electrode, connector, and IPG from the left subclavicular region. The wounds were surgically debrided. The surgery was uneventful, and a swab for microbiological culture was taken. The result revealed the infection caused by Staphylococcus aureus MSSA (methicillin-sensitive). The patient was discharged from the hospital on the 7th day after reoperation in a neurologically stable state, without new complications and with postoperative wounds healing properly. The oral antibiotic Cefadroxil was prescribed, to be taken for 14 days. The patient did not follow the scheduled out-patient visits due to the long distance from her residence to our neurosurgical department. The explanation for this could also be the deterioration of her neurological condition. Moreover, the patient refused to accept the reimplantation of the DBS system on the left side. She had difficulties with ambulation and walked with assistance. The patient was admitted to the nursing facility in December 2023, 18 months after the self-inflicted injury to the left DBS system.
DISCUSSION
Although DBS is a safe and proven treatment modality for patients suffering from debilitating movement and neuropsychiatric disorders, it is not free from complications [11]. These can be grouped into three categories: primary surgery-related, hardware-related and stimulation-induced complications. The surgery-related adverse events are mainly hemorrhagic complications, with a prevalence of less than 5% for all DBS procedures in functional neurosurgery for movement disorders, drug-resistant epilepsy, and neuropsychiatric indications [12, 13]. Most intracranial hemorrhages, due to the introduction of micro- or macro-electrodes as well permanent DBS leads, are clinically silent and the incidence of permanent adverse events reported nowadays is estimated to be below 2%, with a mortality rate below 0.5% of the patients operated on [14]. Stimulation-induced adverse events are found especially in patients treated for treatment-resistant obsessive-compulsive disorder or treatment-resistant depression, less frequently for movement disorders [15]. Hypomania, agitation are very rarely reported as a consequence of STN stimulation, but they can occur in advanced PD after STN DBS [16]. Hypomania may be encountered in patients with PD treated by STN DBS but has more often been related to misplaced DBS leads targeting the anteromedial part of the STN, mainly the limbic rather than sensorimotor part of the STN [16, 17]. In our patient both DBS leads were placed in the dorsolateral motor part of the STN. Moreover, clinical improvement in the presented patient was evident over the course of almost 10 years of continuous bilateral STN DBS, with good control of motor symptoms in off medication condition and amelioration of levodopa-induced dyskinesia in on medication condition as well as on-off fluctuations. We did not observe over this time period any stimulation-induced adverse events related to the bilateral STN DBS itself. These observations suggest that the probably self-injurious behavior was not related to the increase in the daily dose of dopaminergic treatment after STN DBS but rather to the patient’s long-term levodopa intake. In this case the daily levodopa dose before surgery was nearly 1800 mg and dropped to 800/900 mg after bilateral STN DBS. This represents a daily decrease of levodopa intake of nearly 50% when compared to the preoperative daily level. The daily levodopa dose was constant over the 10 years. There was no need to increase it because motor symptoms were well controlled. In the last 3 years, starting from 19 years from diagnosis, axial symptoms like freezing of gait and more frequent falling dominated the clinical picture. This debilitating freezing, noted mostly in the patient’s off-medication condition, resulted in a change from high frequency (130 Hz) to lower frequency (80 Hz) stimulation. This frequency change caused some benefit in ameliorating the freezing and other axial symptoms of PD. Twenty-two years after the diagnosis of PD the patient needed assistance with walking and occasionally used a stick.
The third group of complications include the so-called hardware-related complications. Most of these are the consequences of direct mechanical injury to the implanted DBS system. The damaged parts of the system may be replaced during revision surgeries without subsequent negative sequel for the patients. Most postoperative hardware-related complications after the implantation of the DBS system constitute a mechanical breakage of the extracranial part of DBS lead, connectors, or the malfunctioning of an IPG [18, 19].
The maintenance of an aseptic environment in the operation theater is crucial to avoiding potential contamination and subsequent infection of the implanted hardware. Another factor is how the patient takes care of their wounds after discharge. In our department the policy is to remove stitches 10 days after surgery. Before that the wound is not yet properly healed and remains susceptible to infection. In our department all patients and their caregivers are instructed on how to properly care for postoperative wounds and change dressings to reduce the possibility of infection. The management of skin erosion and infection following DBS surgery constitutes a challenge in the everyday practice and life of patients in the context of having no medical skills, old age, comorbidities, or poor hygiene habits [20]. If a patient notices any redness, edema, or exudation, urgent hospitalization is indicated, with subsequent antibiotics and possible wound debridement. Our patient’s son had good experience in caring for wounds; the patient had had two, mainly uneventful primary surgeries – the staged bilateral implantation of STN DBS and four IPG replacements.
In cases of erosion or visible signs of purulent discharge, the administration of antibiotics should not be delayed. In our patient an intradermal suture was used and, on the 10th, day post-operation the endings were removed by a local general surgeon. The wound in the left subclavicular region over the replaced IPG was properly healed. The patient returned to our neurosurgical department with visible inflammation of the left parietal region, around the IPG and at the left burr hole in the frontal area. She had damaged her left DBS system by breaking the connector. We are not aware of damage being done to the DBS system after a standard exchange of an IPG, especially when the wound over the newly implanted IPG is properly healed – as was reported by the patient’s family members. Also, over the close post-operative period the patient did not complain of itching in the left subclavicular region.
We cannot exclude the possibility of contamination of the replaced left IPG, with a subsequent infection of the entire left DBS system. This situation is rather impossible in the presented case as well in the literature [21]. Early purulent infections are reported to occur usually within one month after the implantation of the entire DBS system than after standard IPG replacement. Our patient had longstanding PD with a 22-year history of a standard daily levodopa dose, consisted of 900 mg over the last ten years. It is estimated that 17% of people taking dopamine agonists experience some degree of compulsive or impulsive behavior, whilst approximately 7% of those taking levodopa and other types of medication are affected [22]. These behaviors are expected to occur with long-term levodopa treatment and in advanced stages of long-standing PD [22].
Cases of damage being done to a DBS system by patients with properly healed wounds are extremely rare. Old age, senile dementia and compulsive-obsessive behavior are additional risk factors for keeping the system operational and intact, particularly for patients living alone. In our patient, as stated above, simple itching was absent, and the family members took special care to see that the wound healed properly. Moreover, simple itching and scrubbing of the wound would result in wound dehiscence with subsequent skin erosion and would have as a consequence infection of the reimplanted DBS hardware. An adverse event such as this would only require the removal of the implantable pulse generator (IPG). In the patient reported on here this was not the case. It is probable that self-injurious behavior and other above-mentioned factors in an old patient with long-standing PD resulted in damage to the entire DBS system, not only the IPG but also the connector and subsequent displacement of the left DBS lead.
The supervision of family members or caregivers of older patients with PD and implanted with DBS hardware may prohibit the scratching of the wounds and damage being done to the DBS system. Postoperative wound care in older patients is mandatory to reduce such infrequent complications. To the best of our knowledge, the case described here is the first in the world literature that presents a case of probably self-injurious behavior causing damage to a DBS system, with infection and subsequent removal. In our patient, this self-harming behavior may be multifactorial but probably resulted from the consequences of long-term levodopa pharmacotherapy [23]; she was suffering from advanced PD with a 22-year history and had been efficiently treated with standard pharmacotherapy and bilateral STN DBS. Our patient met the criteria updated for the advanced stage of PD. In our opinion it is worth mentioning this complication to the neurological and neurosurgical community as a complication found in the late stage of PD [24, 25].
PD represents the second most common neurodegenerative neurological disorder after Alzheimer’s disease and involves not only movement disorders where motor symptoms prevail in the clinical picture but also non- motor symptoms that may become more incapacitating in advanced stages with the coexistence of the cognitive side effects of long-term dopaminergic therapy. Patients with PD, irrespective of pharmacotherapy and advanced forms of treatment like duodopa therapy and DBS, require special supervision by family members or healthcare professionals.
CONCLUSIONS
In this case report we presented a 70-year-old woman with 22-year history of the diagnosis of PD and dopaminergic medication treatment over this period. At the age of 59 the patient underwent staged bilateral STN DBS with good control of Parkinsonian motor symptoms, and a reduction in her daily dose of levodopa. The patient was implanted with single-channel IPGs, which were reimplanted at scheduled hospitalizations at our neurosurgical department. The last subsequent scheduled IPG replacement resulted in self-inflicted damage to the left DBS system, which required removal. To our knowledge, this is the first report of what is probably self-injurious behavior leading to the damage of a DBS system related to the long-standing dopaminergic treatment of PD in a patient in advance stage of this condition.







