INTRODUCTION
Major depressive disorder (MDD) is one of the most common psychiatric diseases associated with increased mortality and shortened lifespan [1]. The prevalence of MDD according to the World Mental Health survey, expressed as a percentage of the whole population, is 14.6% and 11.1% in the high and low-income countries respectively [1, 2]. The classical symptoms of MDD include depressed mood, decrease of energy, decline in interests, loss of interest or pleasure, insomnia or hyper-somnia, psychomotor agitation or retardation difficulties in daily functioning through decreased ability to maintain a job, perform daily activities and function in society, and can even lead to suicide [1]. The total mortality ratio of patients with depression is two times higher than the rest of the population [3].
There are several non-invasive, effective treatments for MDD. The most common conventional MDD treatments are pharmacotherapy, psychotherapy including cognitive-behavioral therapy, and electroconvulsive therapy [4]. Although many patients initially respond to the treatment, there is a percentage who fail to respond, resulting in an estimated prevalence of 1-3% of treatment-resistant depression (TRD) [5]. TRD is established when there is a failure of three established treatment modalities including antidepressants, psychotherapy, and electroconvulsive therapy [3, 5]. TRD is associated with more comorbid mental disorders, a higher number of hospitalizations, and more frequent suicide attempts (30% of patients with TRD attempt suicide) [6]. Patients with TRD show a higher demand for treatment options like vagus nerve stimulation (VNS) and repetitive transcranial magnetic stimulation (rTMS); however, there are no firm recommendations [7-9]. The latest promising but most invasive treatment modality for TRD patients is deep brain stimulation (DBS), which can be the last option for patients who fail to respond to other, less invasive, treatment modalities [10].
THE PIVOTAL ROLE OF THE MEDIAL FOREBRAIN BUNDLE IN MAJOR DEPRESSIVE DISORDER
The medial forebrain bundle (MFB) is the central component of the mesolimbic dopamine reward system that is regarded to be dysfunctional in affective disorders and addiction [11]. The MFB connects multiple brain regions involved in reward processing such as the ventral tegmental area (VTA), lateral and medial hypothalamus, ventral striatum, lateral and medial pre-optic region, nucleus accumbens, septal area, and limbic prefrontal cortex [12]. All these structures are dysfunctional in TRD [12, 13].
The MFB has two distinct tracts: the inferomedial MFB (imMFB) and superolateral MFB (slMFB) [14]. The imMFB follows the lateral wall of the third ventricle into the lateral hypothalamus ending in the olfactory bulb. The slMFB lies beneath the thalamus, moving laterally toward the anterior limb of the internal capsule in its ventral portion that extends into the nucleus accumbens. The slMFB parallels other white matter bundles, mainly the inferior thalamic peduncle (ITP) [14]. Both structures (slMFB and ITP) connect the VTA along the ventral striatum with prefrontal brain areas including the orbitofrontal cortex and dorsolateral pre-frontal cortex [14]. Moreover, some projections from the limbic territory of the subthalamic nucleus (STN) travel within the MFB which activates the hypothalamus and nucleus accumbens, producing the hypomanic or manic states observed in bipolar disorder [14]. This implies the possibility that the MFB may be hyper-active in manic states [15]. The slMFB can be responsible for the activation of nucleus accumbens and associated craving rewards, while the imMFB may produce behavioral responses associated with being pleased with obtained rewards [14, 15].
The MFB has strong connections with well-known brain areas that have been targeted in patients with MDD or TRD [16]. These areas include the anterior limb of the internal capsule, nucleus accumbens, or subcallosal cingu-late cortex [17-21]. These arguments for choosing this neuronal structure as a target in treating TRD have a strong neuroanatomical and functional rationale [13, 14]. DBS at the MFB that is an integrating hub for different targets of the limbic system might produce similar or better antidepressant effects [13, 16].
DEEP BRAIN STIMULATION AS A TREATMENT FOR MAJOR DEPRESSIVE DISORDER
The deep brain stimulation procedure involves the stereotactic implantation of thin electrodes in deep brain structures constituting the elements of the reward circuit to treat major depressive disorder. Up to now, several possible stereotactic targets for DBS in TRD have been identified, including the subgenual anterior cingulate cortex, the ventral capsule/ventral striatum, the nucleus accumbens, the lateral habenula, the anterior limb of the internal capsule, the inferior thalamic peduncle, the medial forebrain bundle, and the bed nucleus of the stria terminalis [22, 23]. The intracerebral electrodes are connected through wires placed subcutaneously in the neck and upper chest region and attached to an implantable pulse generator located in a subcutaneous pocket at the chest wall [24]. Stimulation is generally applied at a high frequency above 100 Hz, usually at 130 Hz, with increasing voltage to 3 or 4 V and pulse width around 60 to 90 microseconds or more, depending on the targeted structure [17, 19, 20, 25]. The whole system implanted in an individual patient is seen in Figure I. The system contains two deep brain stimulation electrodes, two connecting wires, and a single dual-channel or two single-channel implantable pulse generators located subcutaneously in the upper infraclavicular chest region. There are two types of implantable pulse generators: rechargeable and non- rechargeable. Non-rechargeable implantable pulse generators need to be surgically replaced every few years due to battery depletion, whereas rechargeable implantable pulse generators require frequent recharging.
Figure I
Anterior-posterior X-ray of the head, neck, and upper chest region showing the implanted deep brain stimulation system consisting of two intracerebral electrodes (E), connecting wires (CW), and an implantable pulse generator (IPG) for simultaneous stimulation of both hemispheres
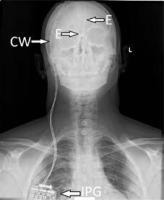
With this background, the DBS of MFB gains importance in the treatment of depression and the evidence regarding its safety and efficacy is rapidly growing. This narrative literature review aims to present clinical outcomes of MFB DBS in the treatment of TRD.
THE CLINICAL TRIALS OF MEDIAL FOREBRAIN BUNDLE DEEP BRAIN STIMULATION FOR MAJOR DEPRESSIVE DISORDER
The first open-label clinical trial of slMFB DBS assessed its safety and efficacy in seven patients with TRD [25]. The stereotactic target was, according to the authors, individualized on the basis of preoperative tractography because it cannot be identified with conventional magnetic resonance imaging [25]. Treatment improvement to bilateral slMFB was fast: six patients improved within 2 days, and four met the criterion for treatment response after one week. At the last follow-up ranging up to 33 weeks, 86% were responders (50% reduction from baseline in the Montgomery-Asberg Depression Rating Scale (MADRS) score) and 57% were remitters (MADRS total score < 10) [25]. These initial antidepressant effects encouraged the authors to present long-term results including one more patient with TRD [26]. At 12 months follow-up, six of eight patients were responders, four of whom were in remission [26]. Some patients were followed up to 50 months with a stable and durable response.
Also, another researcher group investigated the efficacy and safety of slMFB DBS for MDD and the preliminary work data including four patients have been published [27]. One week after surgery the patients entered a single sham stimulation blinded period lasting four weeks, after which they were unblinded for the subsequent 12 months [27]. Within one week of active stimulation, three of the four patients met the criterion of response. One patient was lost in the early postoperative period for further follow-up. At 6 months, two responders continued to improve. This researcher group reported longer follow-up results including two additional patients with TRD [28]. As in the study by Schleapfer et al. and Berwenick et al., the MADRS was used as the primary assessment tool. Deterministic tractography was used to map the slMFB. After one week of stimulation three patients were classified as responders with more than 50% improvement in MADRS scores compared to baseline scores. One patient withdrew from the study. At 52 weeks, the longest follow-up, four of the remaining five patients had more than a 70% improvement in MADRS scores [28]. The authors concluded that the rapid – effective within a week – and strong antidepressant effects are maintained over the ensuing follow-up period, as initially reported by Schlaepfer et al. [25]. The authors supported the use of bilateral slMFB DBS for the treatment of TRD. The largest study to date includes 16 patients with TRD, with encoring results of all patients classified as responders and 50% remitters at the last follow-up visit 12 months postoperatively [29]. Case reports of MFB DBS with 1-2 patients have been conducted, usually with unclear targeting method and lack of surgery description [30-32]. Most of the patients reported in case studies suffered from comorbid obsessive-compulsive disorder or anorexia nervosa [30-33].
In the above-mentioned studies of slMFB DBS, the response and remission rates were based on the Montgomery-Asberg Depression Rating Scale (MADRS) instead of the Hamilton Depression Rating Scale (HDRS) [25-28]. Comparison of MADRS and HDRS postoperative scores may indicate that the use of the MADRS led to a higher proportion of patients meeting the criteria for response and remission. The MADRS is most sensitive in measuring symptom changes as a consequence of treatment [34]. The different outcome measures might have an impact on the outcomes and more unification of depression rating scales between different targets stimulated for TRD is warranted. The clinical outcomes, surgical techniques used and response and remission rates of slMFB DBS are presented in Table 1.
Table 1
A list of published open-label clinical trials and case reports, presented in chronological order, on the utilization of medial forebrain bundle deep brain stimulation (MFB DBS) for the treatment of treatment-resistant depression
| Authors and year of publication | Number of individuals | Main diagnosis | Target/Surgical technique Type of study | Stimulation mode and parameters | Follow-up (months) | Response/remission rate at the final follow-up | Adverse events | Comments |
|---|---|---|---|---|---|---|---|---|
| Schlaepfer et al., 2013 [22] | 7 | TRD One patient with bipolar disorder | Open label case series | Bipolar stimulation 130 Hz 60 µs 2-3 V | 2.8-7.6 months | 87%/57% | One intracranial bleeding due to microrecording | First descriptive clinical case series of slMFB DBS in TRD patients |
| Fenoy et al., 2016 [24] | 4 | TRD | Open-label case series – sham stimulation period of 4 weeks | Bipolar stimulation 130 Hz 60 µs 3 V | 6 months | 75%/25% | No hemorrhagic or infectious complications Light postoperative headache | One patient lost early after surgery |
| Bewernick et al., 2017 [23] | 8 (includes 7 patients previously reported) | TRD | Open label case series | Bipolar 130 Hz 60 µs 2-3 V | 12 months | 75%/50% | This study includes long-term results up to 50 months, from 7 previously published patients Schlaepfer study | |
| Fenoy et al., 2018 [25] | 6 | TRD | Open-label case series – sham stimulation period of 4 weeks | Bipolar 130 Hz 60 µs 3.8 ± 1.2 V | 12 months | 4 of the remaining 5 pts had more than 70% MARDS reduction | Light postoperative headache | 1 patient withdrew early from the study, the non-responder lacked tractography evidence of adequate frontal connectivity to the target |
| Coenen et al., 2019 [26] | 16 | MDD | Phase I clinical study with sham stimulation randomization for 8 weeks | Bipolar 130 Hz 60 µs 3.0 mA (initially 2.1 mA) | 12 months | 100%/50% | One suicide attempt and one hospitalization due to transient hyperkinesia Two infections at the generator sites with subsequent re-implantation. Explantation of the system on patients demand (2) Transient hemiparesis (1) | No difference in sham versus DBS on for the initial 8 weeks |
| Blomstedt et al., 2016 [27] | 1 | AN/MDD | Case report | Bipolar 130 Hz 60 µs 2.8-3.0 V | 24 months | Non-responder | No adverse events | Comorbid OCD AN, unclear definition of the target by tractography |
| Davidson et al., 2018 [28] | 2 | Anhedonic type of MDD | Case series At 6 months double-blind cross-over stimulation off and on phase of 2 weeks’ duration | Bipolar 130 Hz 60 µs 1.5 V (weekly increased by 0.5 till diplopia | 6 months | Both patients regarded as non-responders | No adverse events | Definition of the target unclear |
| Sani et al., 2019 [29] | 1 | MDD | Case report | 140 Hz 60-150 µs 1.5-3.5 V | 6.5 months | Non-responder at 26 weeks | – | Use of novel measurement of depression so-called computerized adaptive test of depression severity |
| Oldani et al., 2017 [30] | 1 | MDD (bipolar disorder) | Case report | Monopolar 0 (–), case (+) 130 Hz 60 µs 1.8 V | 52 months | Full responder | No adverse events reported | Comorbid OCD |
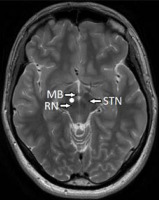
The first authors to introduce MFB DBS have described in detail surgical techniques stressing the importance of implementing preoperative tractography in targeting the slMFB [35]. The authors of these studies emphasized the fact that the slMFB is the first target for DBS defined by tractography [35-37]. Coenen et al. introduced the term therapeutic triangle, as identified on T2 weighted high-resolution magnetic resonance images. The therapeutic triangle (area stimulated) is shown in Figure II and is located between the mammillary bodies, the red nucleus, and the anterior aspect of the subthalamic nucleus. The fibers of the oculomotor nerve are close to this region and transverse the ventral tegmental area laterally. Their stimulation produces often-encountered oculomotor side effects [28-30, 35].
Figure II
Axial T2-weighted brain image at the level of the maximal diameter of the red nucleus. The stimulated region is located between the mammillary bodies, the red nucleus, and the most anterior aspect of the subthalamic nucleus. RN – red nucleus, MB – mammillary body, STN – subthalamic nucleus. The white dot indicates the area targeted and containing the superolateral branch of the medial forebrain bundle (slMFB). The utilization of tractography is mandatory in proper targeting of this white matter bundle constituting the neu-roanatomical substrate of the target
COMPLICATIONS RELATED TO SUPEROLATERAL MEDIAL FOREBRAIN BUNDLE DEEP BRAIN STIMULATION FOR TREATMENT-RESISTANT DEPRESSION
Complications of DBS procedures are divided into three categories, primarily surgery-related, hardware-related, and stimulation-induced complications. Surgery- related complications were minor, usually transient, and without profound impact on the affected patient’s health. There was only one intracranial hemorrhage, which resulted in transient hemiparesis and dysarthria [25]. There were no deaths reported in the literature due to a slMBD DBS procedure in TRD patients [25-33]. There was one suicide attempt and one urgent hospitalization due to stimulation-induced hyperkinesia which resolved after reprograming the stimulation parameters [29]. Two patients were affected by infections at the internal pulse generator site. After infection resolution, new pulse generators were reimplanted [29].
The most common complications in TRD patients who underwent slMFB DBS were stimulation-related and specific to this stimulated brain area [25-29]. The most frequent stimulation-related adverse events were oculomotor disturbances caused by the spread of stimulating current on the oculomotor fibers running medial to the slMFB. Stimulation-related oculomotor symptoms are idiosyncratic for this target region, as the most ventral contact of the implanted DBS lead is close to the ventral tegmental area through which the oculomotor fibers pass [25]. The most common oculomotor adverse events were double vision, blurred vision, and strabismus [25, 29]. To reduce the spread of current most authors used bipolar stimulation mode, narrowing the brain tissue activated [25-29]. The changing of stimulation contacts for more dorsal ones, away from ventral contacts located near oculomotor fibers, also reduces incapacitating oculomotor adverse events. It should be noted that activation of third nerve fibers is very helpful in guiding the implantation itself. Other stimulation-related adverse events are rare and include hypomania, slurred speech, hyperkinesia, impulsivity, and impaired cognition [25-29]. The average stimulation parameters, mode of stimulation, and adverse events related to slMFB DBS are presented in Table 1.
CONCLUSIONS
The clinical trials of slMFB DBS for TRD have delivered some evidence of its acceptable safety and efficacy profile. The main limitation of slMFB DBS is the relatively small number of patients treated. The slMFB DBS has some advantages over previously targeted structures for MDD when compared to the subcallosal cingulate cortex (SCC), nucleus accumbens (NAc), or anterior limb of the internal capsule (ALIC) DBS.
Stimulation of slMFB may have the fastest antidepressant effect (within a week). This effect may potentiate over a follow-up period and is sustainable and durable. Another positive factor are relatively low stimulating parameters, which prolong the duration of internal pulse generators. These two clinical observations might be related to the central role of MFB in the pathogenesis of MDD. The MFB connects frontal areas including the subcallosal cingulate cortex (SCC) to the origin of the mesolimbic dopaminergic reward system in the midbrain ventral teg-mental area.
Emerging technologies, including preoperative tractography of the targeted region, may enhance clinical outcomes. Taking into account postoperative episodes of suicidal ideation and attempted suicides, close postoperative follow-up is mandatory in this very ill and vulnerable patient population incapacitated by TRD. Therefore, slMFB DBS for TRD should only be administered in clinical studies driven by multidisciplinary teams including neurosurgeons, psychiatrists, neuropsychologists, and radiologists. More studies are needed to replicate these encouraging clinical outcomes.






