Introduction
Pancreatic cystic tumors are frequently detected lesions. Small cysts are usually asymptomatic. The rate of incidental detection of pancreatic cysts has been increasing recently due to the increasing sensitivity of imaging studies. The prevalence of pancreatic cysts in the adult population is estimated at 2.6–19.6% [1–3]. High prevalence of pancreatic cysts was also observed in autopsy studies, along with an age-dependent correlation showing the rates of 8% in patients under 70 years of age, and 18%, 30%, and 35% in the 70–79, 80–89, and above 90 age brackets, respectively [4]. On the other hand, magnetic resonance imaging (MRI) scans acquired as part of a prospective, population-based cohort study conducted in a group of 1,077 volunteers in northeastern Germany (Study of Health in Pomerania) revealed the presence of pancreatic cysts in as many as 45.8% of the subjects (cysts sized < 5 mm in 63.6% of subjects, cyst sized 5–10 mm in 30.6% of subjects, cysts sized 10–20 mm in 5.1% of subjects and cysts sized > 20 mm in 0.7% of subjects). In a 5-year follow-up, new pancreatic cysts were identified in 12.9% of the subjects, while the size of previously detected cysts increased by an average of 24% [5].
The most common (> 90%) types of pancreatic cystic tumors diagnosed in patients subjected to surgical treatment include intraductal papillary mucinous neoplasm (IPMN) – about 45% of cases; mucinous cystic neoplasm (MCN) – about 18% of cases; serous cystic neoplasm (SCN) – about 16% of cases; solid pseudopapillary neoplasm (SPN) – about 6% of cases; and cystic neuroendocrine neoplasm (NEN) – about 5% of cases [6, 7].
About 60–70% of cases of intraductal papillary mucinous neoplasm (IPMN) are diagnosed in male patients aged about 60–70 years. IPMN originates from the muciferous epithelial cells and forms papillary thickenings within the pancreatic duct. It is characterized by dilatation of the main pancreatic duct or by the presence of cysts derived from the dilated branches thereof. In advanced stages, the presence of mucus is observed in the ampulla of Vater (the sign being referred to as fish-eye ampulla) in endoscopic examinations. The distinct types of IPMN include main duct IPMN (IPMN-MD), branch duct IPMN (IPMN-BD), and mixed-type IPMN. Histological phenotypes of IPMN include the gastric phenotype – most common in IPMN-BD, characterized by low risk of malignant transformation – and the intestinal phenotype, common in IPMN-MD and presenting with a higher risk of malignant transformation compared to other IPMN types [8]. A pancreatobiliary phenotype of a highly aggressive course is also detected in rare cases.
The mucinous cystic neoplasm (MCN) is detected mostly in women (20:1) of premenopausal age. Tumors are usually isolated and localized in the body or tail of the pancreas. They are made up of thick-walled, multi-chambered cysts that do not communicate with the pancreatic ducts. The cyst chambers are 1–3 cm in diameter and filled with mucous contents. The cysts have thick walls, and progesterone and estrogen receptor-expressing cells are present within the cyst lining [9, 10].
Serous cystic neoplasm (SCN) is a benign neoplasm composed of glycogen-rich epithelial cells that form numerous small cysts filled with serous contents and combining to form large tumor-like lesions. This type of lesion usually affects patients between the ages of 61 and 65. SCN is more common in women than in men (3 : 1). Most cysts are located within the body and tail of the pancreas. The cross-section shows numerous, tightly packed, small, thin-walled cysts of sponge-like or honeycomb presentation, organized around a central scar with calcifications. The lesions do not communicate with the pancreatic ducts [11–14].
The risk of developing cancer from a pancreatic cyst depends on the nature of the lesion, its size, and the presence of factors that further increase the risk, such as a positive family history of pancreatic cancer [15, 16]. The risk of developing pancreatic cancer is highest for IPMN and MCN [17, 18], and therefore the identification of the mucinous character of the pancreatic cysts is of crucial importance. Oncological vigilance should be maintained for all pancreatic cysts of unclear etiology. Over the past several years, numerous guidelines have been published on the diagnostic and therapeutic management of pancreatic cystic tumors (Sendai 2006; Fukuoka 2012; the 2015 American Gastroenterological Association Guidelines; the 2018 American College of Gastroenterology Guidelines; the 2018 European Guidelines, and, more recently, the 2023 Hong Kong Guidelines and the 2024 Kyoto Guidelines) [11, 19–23]. Experts of the Polish Pancreatic Club (Polski Klub Trzustkowy – PKT) undertook the task of developing Polish guidelines based on international recommendations published to date and taking into account the latest scientific reports on the pathophysiology and natural history of pancreatic cystic tumors. The certainty (quality) of the evidence and the strength of the recommendations were assessed on the basis of the GRADE methodology (Tables I and II), and the degree of acceptance of individual statements as determined from the results of the voting as held by PKT experts using the 6-point Likert scale shown in Table III [24].
Table I
Criteria for evaluating the certainty (quality) of evidence according to the GRADE system
Table II
Criteria for evaluating the recommendation strengths according to the GRADE system
Statements
Diagnostics
1. Pancreatic cysts of unclear etiology, as identified in imaging studies, require complementary diagnostics to be carried out in specialized reference centers.
| Quality of evidence: moderate. |
| Recommendation strength: strong. |
| Total support: 59.1%, Support: 18.2%, Partial support: 22.7%, Partial objection: 0%, Objection: 0%, Total objection: 0%. |
If a cystic lesion of the pancreas of unclear etiology is found, it is recommended that further diagnosis be carried out at a reference center with a qualified multidisciplinary team. Notably, such management should be applied not only to patients with cystic lesions requiring surgical resection, but also to patients with cysts showing worrisome features [16, 21, 23]. Some of these patients are not deemed eligible for surgical resection following a careful evaluation of the balance of benefits of cyst surveillance versus the decision to operate. It is important that the patient be included in the decision-making process; patients with cystic tumors should be informed of the potential risks and benefits of surgical treatment vs. surveillance. Moreover, the involvement of a multidisciplinary group of pancreatic specialists was shown to result in potential changes to patient management, including surgery being replaced by watchful surveillance in 30% of patients [16, 21, 25–28]. Proper surveillance and treatment of pancreatic cystic tumors requires a skilled multidisciplinary team, as well as access to specialized diagnostic methods.
2. Abdominal ultrasonography (US) is not sufficient to establish the character of the cyst. Contrast-enhanced computed tomography (CT) or contrast-enhanced magnetic resonance imaging (MRI) is required for further diagnostic evaluation.
| Quality of evidence: low. |
| Recommendation strength: strong. |
| Total support: 72.2%, Support: 18.2%, Partial support: 9.1%, Partial objection: 0%, Objection: 0%, Total objection: 0%. |
The main imaging studies used for the detection of pancreatic cysts are ultrasound, CT, and MRI scans. However, abdominal ultrasound usually facilitates only the assessment of the size and the approximate location of the cyst [29]. Contrast-enhanced abdominal CT and MRI scans (1.5 T or 3 T) facilitate differentiation of the character of the cyst, determination of probable diagnosis, and identification of the presence of “worrisome features”. MRI appears to be more sensitive than CT when assessing communication between the cyst and the pancreatic duct, the presence of enhancing wall nodules, and the presence of intracystic septa. The CT scans, on the other hand, facilitate better assessment of intracystic calcifications and possible vascular infiltrations as compared to MRI scans [22, 29–31]. MRI is recommended for long-term surveillance to avoid the risk of exposure to ionizing radiation as used in CT [22].
3. Endoscopic ultrasound (EUS) is necessary to confirm the diagnosis in cases of pancreatic cysts of uncertain character.
| Quality of evidence: moderate. |
| Recommendation strength: strong. |
| Total support: 59.1%, Support: 31.8%, Partial support: 9.1%, Partial objection: 0%, Objection: 0%, Total objection: 0%. |
Endosonography is a complementary method for use in differentiation of the nature of pancreatic cysts. The modality ensures high-resolution images allowing for accurate determination of the size and the number of cysts, their communication with the pancreatic duct and the potential presence of parietal nodules within the cyst walls [21, 22]. This facilitates the identification of potential “worrisome features” or “high-risk stigmata” for easier qualification of patients for surgical treatment [23]. In addition, the diagnosis can be expanded by contrast-enhanced ultrasound evaluation of solid cyst contents. The presence of enhancing parietal nodules may indicate the site for a biopsy of the cystic lesion to be collected under EUS guidance, while also being one of the elements suggesting the need for surgical treatment [21, 23]. Targeted biopsies of pancreatic cystic lesion are also possible under endosonographic guidance.
4. EUS-guided biopsy is a safe and useful method to determine the character of pancreatic cysts.
| Quality of evidence: moderate. |
| Recommendation strength: strong. |
| Total support: 59.1%, Support: 36.4%, Partial support: 4.5%, Partial objection: 0%, Objection: 0%, Total objection: 0%. |
EUS-guided biopsy of a pancreatic cyst is recommended when its character is unclear from CT or MR imaging. Biopsy facilitates differentiation between mucinous and non-mucinous cystic tumors and increases the likelihood of detecting cysts with malignant transformations [21]. A biopsy should be performed whenever its expected outcome would affect further management, such as deciding between surgery and continued surveillance.
EUS-guided biopsy of pancreatic cysts is a relatively safe method, the risk of complications amounting to about 3.4%. The observed complications are usually mild and include post-procedural pain (34%), acute pancreatitis (34%), infectious complications (16%), bleeding (13%), and perforation (3%) [32–35]. Antibiotic prophylaxis using fluoroquinolones or beta-lactam antibiotics is recommended with pancreatic cyst biopsy [36]. The risk of potential tumor dissemination as a result of EUS-guided biopsy is minimal, about 0.3% according to one meta-analysis [37].
5. Pancreatic cystic adenocarcinoma cannot be excluded on the basis of the absence of tumor cells in the cytological examination of pancreatic cyst fluid.
| Quality of evidence: low. |
| Recommendation strength: strong. |
| Total support: 81.8%, Support: 18.2%, Partial support: 0%, Partial objection: 0%, Objection: 0%, Total objection: 0%. |
Pancreatic cyst fluid collected during EUS-guided biopsy usually contains a sparse number of cells, translating to the low sensitivity of cytological examinations of pancreatic cyst fluid. The sensitivity and specificity of cytological examinations as determined from meta-analyses are in the range 51–63% and 88–94%, respectively [38, 39]. Biopsy of any solid content of pancreatic cysts is recommended to obtain tissue material for the diagnosis of dysplasia or cancer.
Macroscopic evaluation and mucus staining of the collected cyst fluid are also important [40, 41]. Determining the character of a pancreatic cyst usually requires comprehensive evaluation, with the clinical data, the morphology of the pancreatic cyst, the results of the cytological examination, and the biochemical assays of the cyst fluid (CEA, amylase, glucose) being taken into account.
6. Currently available biochemical pancreatic cyst fluid assays (amylase, CEA, glucose) are helpful in differentiating between mucinous and non-mucinous cysts. However, they are not useful for the detection of a malignant process within the cyst.
| Quality of evidence: moderate. |
| Recommendation strength: strong. |
| Total support: 68.2%, Support: 27.3%, Partial support: 4.5%, Partial objection: 0%, Objection: 0%, Total objection: 0%. |
Assessment of the levels of carcinoembryonic antigen (CEA) and glucose as well as the activity of amylase within the pancreatic cyst fluid facilitates differentiation between mucinous and non-mucinous cysts. CEA levels of above 192 ng/ml correlate with the diagnosis of cystic mucinous tumor with a sensitivity of 63% and specificity of 88% [35].
Low activity of amylase in the pancreatic cyst fluid facilitates the diagnosis of a pseudo-cyst being excluded with a high likelihood. However, high activity of amylase in the pancreatic cyst fluid does not allow any conclusions regarding the character of the pancreatic cyst [42]. Pancreatic cyst fluid glucose levels of 50 mg/dl are highly sensitive in the diagnosis of mucinous cysts. According to meta-analyses, this method has a sensitivity of 90.1% and a specificity of 85% [43, 44].
7. The presence of at least one of the high-risk stigmata – mechanical jaundice caused by the cystic tumor, main pancreatic (Wirsung’s) duct being dilated to > 10 mm, or an enhancing intracystic mass within a cyst sized > 5 mm – is highly indicative of the presence of high-grade dysplasia or invasive carcinoma within the pancreatic cystic tumor.
| Quality of evidence: moderate. |
| Recommendation strength: strong. |
| Total support: 68.2%, Support: 31.8%, Partial support: 0%, Partial objection: 0%, Objection: 0%, Total objection: 0%. |
Mechanical jaundice caused by the cystic tumor, main pancreatic (Wirsung’s) duct being dilated to > 10 mm, an enhancing intracystic mass of > 5 mm, and the detection of suspicious or neoplastic cells in cytological examination of fluid obtained from the pancreatic cyst are considered high-risk stigmata [21–23]. The presence of mechanical jaundice caused by a pancreatic cystic tumor indicates, with high probability, the presence of high-grade dysplasia (HGD) or invasive carcinoma (IC) – sensitivity of 75–83%, specificity of 61–65% [45–47]. According to data reported in the 2018 European guidelines, a pancreatic cyst with the presence of a parietal, enhancing nodule sized over 5 mm is indicative of HGD/IC with a sensitivity of 73–100% and specificity of 73–85% [21]. Current data, however, indicate that the presence of a parietal nodule over 5 mm in diameter alone, without any other high-risk stigmata or worrisome features of concern, is associated with a moderately increased risk of HGD/IC – OR 1.19–3.16 [48–50]. Dilatation of the main pancreatic duct to > 10 mm with no other worrisome features is also associated with a slight increase in the risk of HGD/IC – OR 1.06–1.76 [48–50]. However, the authors of the Kyoto 2023 Guideline conclude that co-occurrence of the main pancreatic duct dilatation of > 10 mm and the presence of enhancing parietal nodules sized > 5 mm should be defined as a high-risk stigma [23]. Detection of cells presenting with high-grade dysplasia in cytological examination of pancreatic cyst fluid is associated with a very high probability of HGD/IC – sensitivity of 91–100%, specificity of 100% [51].
8. The presence of worrisome features, such as acute pancreatitis, elevated serum CA19-9 levels, newly diagnosed diabetes mellitus, cyst diameter greater than 30 mm, enhancing intracystic tissue masses in cysts sized < 5 mm, thickened, enhancing cyst walls, main pancreatic (Wirsung’s) duct dilatation to 5–9 mm, enlarged lymph nodes, or cyst size increasing by > 2.5 mm within a year may be suggestive of the presence of high-grade dysplasia or invasive carcinoma within the pancreatic cystic tumor. The co-occurrence of several worrisome features significantly increases this probability.
| Quality of evidence: moderate. |
| Recommendation strength: strong. |
| Total support: 77.3%, Support: 18.2%, Partial support: 4.5%, Partial objection: 0%, Objection: 0%, Total objection: 0%. |
Worrisome features are defined as: (1) acute pancreatitis caused by a cystic tumor; (2) elevated serum CA19-9 levels; (3) diabetes mellitus diagnosed within the last year; (4) cyst diameter larger than 30 mm; (5) enhancing parietal nodules sized < 5 mm; (6) thickening or enhancement of the cyst wall; (7) main pancreatic duct diameter of ≥ 5 mm and < 10 mm; (8) abrupt change in the diameter of the pancreatic duct with atrophy of the distal part of the pancreas; (9) lymphadenopathy; (10) cyst growth rate of ≥ 2.5 mm/year [23].
The main mechanisms leading to acute pancreatitis in IPMN patients include the obstruction of the main pancreatic duct due to high mucus viscosity or the narrowing of the pancreatic duct due to tumor compression. Acute pancreatitis occurs with similar frequency in both IPMN with HGD/IC and low-grade dysplasia (LGD) [50]. Regardless of the grade of dysplasia in the course of IPMN, recurrent episodes of acute pancreatitis and associated deterioration in the quality of life may be an indication for surgical treatment.
Elevated serum CA19-9 levels (> 37 U/l) are a predictor of various gastrointestinal cancers, including pancreatic adenocarcinoma, as well as IPMN with IC, with a sensitivity of 41–74% and a specificity of 85–96% [48]. Current data suggest a 25% prevalence of newly diagnosed diabetes in IPMN patients, the feature being associated with an increased risk of HGD (OR = 1.27) and IC (OR = 1.61) [52–55]. On the other hand, cyst wall thickness of ≥ 2.5 mm as assessed by EUS and the contrast enhancement thereof are indicative of an increased risk of HGD/IC (OR = 3.51) [23]. An abrupt change in the diameter of the pancreatic duct with atrophy of the distal part of the pancreas and lymphadenopathy have been included among the worrisome features the Kyoto Guidelines, despite the lack of high-quality evidence to support these observations [23].
Recent studies have shown that an average increase in the size of the IPMN cyst by 2.5 mm/year is a significant predictor of progression to HGD/IC [56–58]. This parameter was included among the worrisome features in the Kyoto Guidelines [23]. However, it is important to note that discrepancies exist in the assessment of cyst sizes between different imaging modalities as well as between individual investigators.
The presence of several worrisome features increases the risk of HGD/IC. The risk of HGD/IC increases progressively with the number of worrisome features: up to 22%, 34%, and 59% with one, two, and three worrisome features, respectively, reaching 100% in patients presenting with four or more worrisome features [59, 60].
Treatment
9. Surgical treatment of pancreatic cysts should be carried out at specialized centers with extensive experience in pancreatic surgery.
| Quality of evidence: low. |
| Recommendation strength: strong. |
| Total support: 90.9%, Support: 9.1%, Partial support: 0%, Partial objection: 0%, Objection: 0%, Total objection: 0%. |
In cases of pancreatic cysts being identified as requiring surgical resection, the treatment should be carried out by an experienced surgical team at a high-volume pancreatic surgery center. The mortality rate was shown to be nearly three times higher when pancreatoduodenectomy is performed by an inexperienced surgeon at a low-volume center as compared experienced surgeons at high-volume centers (11–15% vs. 1–5%) [61, 62]. According to the guidelines by the Leapfrog Group, a health care quality improvement organization, it is recommended that surgeons performing pancreatoduodenectomies carry out at least 15 procedures of this type per year. In addition, the respective medical center should perform a minimum of 40 procedures of the same type per year [63].
Other studies confirm that centers performing fewer than 5 pancreatic procedures per year have higher mortality rates compared to facilities carrying out at least 40 procedures per year [64]. High-volume pancreatic surgery centers achieve significantly better outcomes, such as fewer complications, lower postoperative mortality, and longer patient survival [65]. Better outcomes are the result of greater clinical experience, better-organized medical teams, and developed care procedures.
10. The presence of clinical symptoms caused by the tumor lesion, such as mechanical jaundice, gastrointestinal obstruction, at least one of the high-risk stigmata for HGD/IC, or the presence of several worrisome features, is an indication for surgical treatment of a pancreatic cystic tumor.
| Quality of evidence: moderate. |
| Recommendation strength: strong. |
| Total support: 63.6%, Support: 31.8%, Partial support: 4.5%, Partial objection: 0%, Objection: 0%, Total objection: 0%. |
Patients with clinical symptoms caused by a cystic tumor (e.g., mechanical jaundice, obstruction of the gastrointestinal tract) require surgical treatment without the diagnostic process being extended to determine the character of the cyst [21, 23].
Identification of at least one high-risk stigma (HRS) is associated with a high risk of HGD/IC in the diagnosed lesion, although the predictor was not shown to be of perfect specificity [23]. It is recommended that surgical treatment decisions be made very carefully and not only on the basis of HGD/IC prediction. Instead, the patient’s overall condition, comorbidities, life expectancy and the patient’s informed consent should also be taken into account. Therefore, the terms “high-risk stigmata” (HRS) and “worrisome features” (WF) continue to be used in the current guidelines instead of “absolute indication” and “relative indication” for surgery [23] (Table IV).
Table IV
Worrisome features
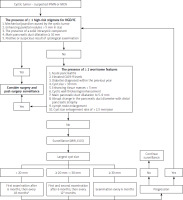
Identification of at least one high-risk stigma of high-grade dysplasia or carcinoma (HGD/IC) (Table V) is an indication for surgery [21–23] (Figure 1). The overall risk of HGD or IC is four to six times higher for the presence of parietal nodules, with a positive predictive value of 60% [66]. Based on the studies, the cutoff point of > 5 mm was established for the size of an enhancing parietal nodule as a predictor of HGD/IC with a sensitivity of 73–100% and a specificity of 73–85% [23, 66]. The risk of HGD/IC increases along with the increase in the dimensions of the parietal nodule [23].
Table V
High-risk stigmata for HGD/IC
According to some reports, about 10% of malignant BD-IPMN present with no parietal nodules. Nearly all of these cases are those of HGD, warranting a conclusion that invasive cancers are rare in the absence of parietal nodules within the cyst [66–68]. Note should also be taken of the result of the cytological examination, since a “suspicious” or “positive” (that is, confirming the presence of predicted malignant lesions) image is considered a high-risk stigma of HGD/IC [23].
A separate discussion is required regarding the four worrisome features accompanying the cystic tumors as listed in the table. It should be emphasized that the presence of any one of these features increases the risk of a neoplastic process within the observed lesion, while the occurrence of several (≥ 2) features is an indication to consider surgical treatment [21–23] (Figure 1).
Acute pancreatitis (AP) in IPMN patients is caused by the obstruction of the main pancreatic (Wirsung’s) duct due to the secretion of viscous mucus. Another mechanism behind AP consists in the main pancreatic duct narrowing as a result of being compressed by the tumor. Notably, in addition to affecting the prognosis of IPMN dysplasia, AP may adversely affect the patient’s quality of life. Therefore, surgery should be considered in patients with recurring episodes of acute pancreatitis against the background of a cystic tumor [23].
Counted among the worrisome features, the elevation in serum CA19-9 levels (> 37 U/l), predicts IC in the course of IPMN with a sensitivity of 63.3%, specificity of 78.0%, and accuracy of 73.3% [69]. The use of serum CEA levels in similar differentiation is significantly limited by the far lower sensitivity of this parameter, reported to be as low as 18% [70].
The recently published guidelines and studies on cystic tumors highlight newly diagnosed diabetes and sudden deterioration in glycemic control in the course of previously diagnosed diabetes as worrisome features [22, 23]. Newly diagnosed diabetes is common (25%) in IPMN patients and increases the risk of HGD (risk ratio = 1.27) as well as IC (risk ratio = 1.61) [55].
The thickening/enhancement of the cyst wall and possible intracystic septa is an ambiguous factor that has not been fully evaluated as a predictor of HGD/IC. As reported by the authors of one study, the odds ratio for HGD/IC prediction is 3.51 when the septal thickness as assessed by EUS is ≥ 2.5 mm [71].
The cystic tumor growth rate is also a clinically important factor. Based on the observations, an increase of 2.5 mm/year could be a predictor of HGD/IC progression in IPMN patients [22, 23]. On the other hand, the lesion imaging modality and the possible influence of subjective image interpretation should be taken into account when evaluating and comparing this parameter [72].
11. Confirmation of high-grade dysplasia or adenocarcinoma on microscopic examination of biopsy material is an indication for surgical treatment of pancreatic cystic tumor.
| Quality of evidence: moderate. |
| Recommendation strength: strong. |
| Total support: 68.2%, Support: 31.8%, Partial support: 0%, Partial objection: 0%, Objection: 0%, Total objection: 0%. |
The finding of HGD in a cystic tumor biopsy offers a unique opportunity for surgical cure as the lesion immediately precedes the onset of pancreatic cancer [73]. According to the International Cancer of the Pancreas Screening Consortium, surgical removal of HGD helps in the prevention of pancreatic cancer [74]. According to observations, the time required for HGD in a cystic tumor to transform into invasive cancer is 3 years [75], thus providing an opportunity for early detection and intervention.
The result of cytological (or, in selected cases, histopathological) examination confirming high-grade dysplasia or cancer is an indication for surgical treatment in patients whose general condition facilitates such an approach. Informed consent should be obtained from the patient prior to the surgical treatment, and the decision should be made with the concomitant diseases as well as the patient’s life expectancy and quality of life being taken into account.
12. Adjuvant chemotherapy is beneficial in most patients with resectable ductal carcinoma resulting from a malignant transformation of a pancreatic cystic lesion.
| Quality of evidence: moderate. |
| Recommendation strength: strong. |
| Total support: 59.1%, Support: 40.9%, Partial support: 0%, Partial objection: 0%, Objection: 0%, Total objection: 0%. |
Adjuvant systemic chemotherapy is recommended for IPMN accompanied by invasive cancer with or without lymph node involvement. No strong scientific evidence is available to demonstrate the benefits of adjuvant chemotherapy in patients without lymph node involvement [76], while the approach may be warranted by the aggressive course of clinical pancreatic cancers [77–79]. Adjuvant treatment of MCN-associated invasive cancer is similar to that of sporadic pancreatic adenocarcinoma [21].
Current evidence suggests that adjuvant chemotherapy in invasive IPMN should be used in a selective manner, focusing on patients at higher risk of recurrence and with worse prognosis. Studies confirm that patients with lymph node metastases, positive resection margins, poorly differentiated tumors, and higher TNM stages benefit the most [80–83]. In these patients, adjuvant therapy significantly improves overall and disease-free survival, especially in cases of tubular type carcinomas and elevated CA19-9 levels, suggesting that these markers may be helpful in identifying patients who should receive postoperative treatment.
At the same time, the early stage of the disease (stage I) and the absence of lymph node metastases do not warrant the use of adjuvant therapy, as studies indicate that the benefits of chemotherapy are minimal or absent in these cases [81]. This is explained by the less invasive course of IPMN as compared to classic pancreatic ductal adenocarcinoma (PDAC) and indicates the need for a cautious approach to treatment so as to prevent overtreatment and the associated side effects [83].
13. The currently available study data do not warrant a recommendation for neoadjuvant treatment of locally advanced invasive cancer associated with IPMN or MCN.
| Quality of evidence: low. |
| Recommendation strength: weak. |
| Total support: 50.1%, Support: 40.9%, Partial support: 4.5%, Partial objection: 0%, Objection: 4.5%, Total objection: 0%. |
Given the lack of sufficient data, no clear recommendations can be made regarding neoadjuvant treatment of locally advanced invasive cancer associated with IPMN or MCN [21]. The potential feasibility of such an approach is supported by only a few case reports on the use of preoperative chemotherapy in IPMN and MCN patients [84, 85]. Instead, an approach similar to that used in patients with pancreatic cancer can be considered, given the similarities between the two diseases [21].
14. Palliative chemotherapy may be considered in patients with inoperable, recurrent, or metastatic malignant IPMN or MCN, by analogy to pancreatic cancer.
| Quality of evidence: low. |
| Recommendation strength: weak. |
| Total support: 50.0%, Support: 50.0%, Partial support: 0%, Partial objection: 0%, Objection: 0%, Total objection: 0%. |
Palliative chemotherapy may be considered for inoperable or recurrent malignant cystic tumors by analogy to pancreatic adenocarcinoma. Although a sufficient body of published data is not available to unambiguously support such an approach, palliative chemotherapy in patients with inoperable, recurrent or metastatic disease in the course of malignant IPMN or MCN appears to have similar treatment outcomes as in pancreatic cancer [21, 86].
Single case reports have confirmed favorable responses to regimens including gemcitabine, nab-paclitaxel, and FOLFIRINOX, resulting in significant progression-free periods and extended overall survival [87].
In some cases of MCN presenting with BRCA1 mutations, PARP inhibitors such as olaparib were shown to be efficient in controlling the disease after the initial lines of chemotherapy, thus opening up additional therapeutic options [88]. As shown by the above examples, multi-line chemotherapy, including platinum-based or PARP inhibitor-based (in the case of relevant mutations) regimens, can provide a valuable palliative strategy, offering options to prolong survival and improve treatment quality in patients with inoperable malignant cystic tumors.
15. Treatment of neoplastic pancreatic cysts using ablative methods, including ablation with ethanol or paclitaxel infusions, is not recommended. Also, endoscopic procedures have no use in the treatment of pancreatic cystic tumors beyond palliative management.
| Quality of evidence: low. |
| Recommendation strength: strong. |
| Total support: 59.1%, Support: 27.3%, Partial support: 9.1%, Partial objection: 0%, Objection: 0%, Total objection: 4.5%. |
Patients diagnosed with IPMN and meeting the criteria for surgical resection while being ineligible for the surgery present a serious therapeutic dilemma. In the last two decades, reports have been published on the possibility of using ablative techniques, such as ethanol injections, administration of paclitaxel [89, 90], or radiofrequency ablation under EUS guidance [91], in these patients.
Preferred candidates for ablative therapy include patients with suspected or confirmed mucinous cystic lesions that are unilocular or oligolocular, patients with enlarging pancreatic cysts sized > 2 cm in diameter, or patients with pancreatic cysts sized > 3 cm in diameter and not communicating with the main pancreatic duct. Some of the relative contraindications to ablation include cysts with enhancing parietal nodules, cysts with no or low malignant potential, main pancreatic duct dilated to > 5 mm, obvious open communication between the cyst and the main pancreatic duct, thick cystic walls or intracystic septa, significant solid components inside the cysts, and history of acute pancreatitis [92].
Studies show that the success rates of ablation procedures range from 9% to 79% [92, 93]. However, long-term follow-up data on survival and recurrence risk reduction are limited. In addition, technical difficulties – namely, the inability to accurately visualize the cyst after its collapse – pose a significant challenge to assessing the efficacy of ablation [66]. The most commonly reported complications include abdominal pain (up to 25%), acute pancreatitis (about 10%), splenic vein obstruction, and peritonitis [92]. Cases of pancreatic ductal adenocarcinoma (PDAC) developing at sites independent of the primary cystic lesions have also been described [94], suggesting that the ablative procedure may not satisfactorily reduce the risk of cancer.
In conclusion, EUS-guided ablative techniques for the treatment of IPMN require further research, especially regarding their safety, efficacy, and long-term oncological outcomes. Based on data from most publications and guidelines on the management of cystic tumors [11, 21, 23, 66, 95], ablative techniques cannot be currently recommended for routine practice and should only be used within the framework of closely monitored clinical trials.
16. Pancreatic cancer cysts without concurrent exocrine pancreatic insufficiency are not an indication for pancreatic enzyme supplementation.
| Quality of evidence: low. |
| Recommendation strength: strong. |
| Total support: 81.8%, Support: 13.6%, Partial support: 4.5%, Partial objection: 0%, Objection: 0%, Total objection: 0%. |
Exocrine pancreatic insufficiency (EPI) is the pancreatic inability to deliver the required threshold levels of digestive enzymes to the intestine for the digestion of food and to meet nutritional and metabolic needs [96]. Symptoms of EPI vary depending on the severity of the failure and may include steatorrhea, weight loss, nausea, bloating, recurrent abdominal pain (worsening after eating), and symptoms of micronutrient and fat-soluble vitamin deficiencies (e.g., osteopenia, osteoporosis). In advanced, untreated forms, EPI leads to malnutrition and cachexia [97, 98].
At present, no conclusive study data are available to demonstrate the direct effects of the presence of a cystic tumor on the exocrine capacity of the pancreas. However, various lesions, including cancerous lesions within the pancreas, are known to be capable of leading to exocrine failure. When the cyst is located proximally (e.g., near the head of the pancreas), mechanical disturbances in the flow of pancreatic juice containing pancreatic enzymes can be expected, theoretically affecting the exocrine function [99]. Another mechanism responsible for the potential development of EPI in patients with a cystic tumor involves potential episodes of acute pancreatitis as a consequence of the presence of the cyst itself [100]. Regardless of the proposed hypotheses explaining the occurrence of EPI in patients with pancreatic cystic tumors, it should be emphasized that the mere presence of pancreatic cysts does not constitute an indication for pancreatic enzyme replacement therapy. When clinical signs that may suggest EPI are observed, it is advisable that the concentration of elastase-1 in the stool be determined. A result of less than 200 µg/g indicates mild exocrine pancreatic insufficiency, while a value of less than 100 µg/g indicates severe exocrine pancreatic insufficiency.
A separate issue consists in the possibility of EPI developing in patients following pancreatic cystic tumor resection. The risk of EPI depends on the type of surgery and the extent of the resected pancreatic parenchyma. The incidence of EPI after distal pancreatectomy ranges from 19 to 50% and depends mainly on the volume of remaining pancreatic parenchyma, as well as the type of disease responsible for the surgery. For the Whipple procedure, the rate of EPI can range from 56 to as high as 98% [101]. Every patient who has undergone pancreatic resection surgery should be evaluated for possible exocrine pancreatic insufficiency and, if confirmed, subjected to appropriate enzyme replacement therapy [102, 103].
17. Patients after partial pancreatectomy should be screened for diabetes, and the subsequent management should follow current standards of diabetes care.
| Quality of evidence: low.. |
| Recommendation strength: strong. |
| Total support: 63.6%, Support: 27.3%, Partial support: 9.1%, Partial objection: 0%, Objection: 0%, Total objection: 0%. |
The results of a systematic review evaluating the incidence of type 3c diabetes in patients who have undergone various pancreatic resection procedures show that diabetes is diagnosed in 9–24% of patients after pancreatoduodenectomy, 3–40% of patients after distal pancreatic resection, and 0–14% of patients after central pancreatic resection [104]. Diabetes develops in all patients after total pancreatic resection [105]. The risk of developing diabetes depends not only on the type of surgery and the volume of remaining pancreatic parenchyma, but also on preoperative HbA1c levels and fasting glucose levels [104].
Diabetes screening is recommended in patients who have undergone pancreatic resections for cystic tumors [22, 106]. If the first screening result is negative, regular monitoring of glucose levels and other metabolic indicators is recommended. This management is justified by observations showing that diabetes can develop many months after the surgery, the risk of diabetes being the highest in the first year after surgery [107]. Follow-up examinations are usually conducted at regular intervals, usually every 3 to 6 months in the first year after the surgery, and every 6 to 12 months thereafter, depending on the results of the initial screening and the patient’s individual risk factors [107].
Surveillance
18. The surveillance of patients after surgical treatment of a pancreatic cystic tumor and histopathological confirmation of invasive adenocarcinoma should follow the existing guidelines for pancreatic cancer.
| Quality of evidence: moderate. |
| Recommendation strength: strong. |
| Total support: 77.3%, Support: 18.2%, Partial support: 4.5%, Partial objection: 0%, Objection: 0%, Total objection: 0%. |
As shown in the studies, disease recurrence occurs in up to 43% of patients operated on for IPMN with confirmed IC [108]. After the surgical treatment of a pancreatic cystic tumor and histopathological confirmation of invasive adenocarcinoma, further surveillance should include monitoring for possible recurrence, the assessment of side effects of treatment, and support in patient’s rehabilitation and quality of life improvement [109]. Surveillance should include clinical assessment and symptom monitoring at regular follow-up visits scheduled every 3 to 6 months for the first year and every 6 months for the second year after resection; the subsequent evaluations may be less frequent (every 12 months). In addition, a contrast-enhanced CT or MRI scan of the abdomen is acquired. The CA 19-9 levels are measured regularly (every 3 to 6 months) in order to assess the efficacy of treatment and to detect any recurrence early [109, 110].
Following pancreatic resection due to neoplastic lesions, patients are at high risk for eating disorders, potentially leading to malnutrition. In such cases, dietary support, appropriate diagnostics, and supplementation of pancreatic enzymes and vitamins are essential [111].
19. The frequency of surveillance after surgical treatment of a pancreatic cystic tumor and histopathological confirmation of a non-invasive malignant lesion depends on the existing risk factors. If at least one risk factor (HGD or family history of pancreatic cancer) is identified, surveillance should be conducted every 6 months as opposed to every 12 months in the absence of the above risk factors.
| Quality of evidence: low. |
| Recommendation strength: strong. |
| Total support: 45.5%, Support: 40.9%, Partial support: 13.6%, Partial objection: 0%, Objection: 0%, Total objection: 0%. |
The frequency of postoperative surveillance in patients after partial pancreatic resection due to a cystic tumor with histopathological confirmation of the noninvasive nature of the neoplastic lesion depends on the status of certain risk factors, such as the presence of HGD or a family history of pancreatic cancer [23]. This approach is justified since clinically significant lesions can develop in the remaining parts of the pancreas even after partial pancreatectomy, despite the lesion being removed with negative resection margins [112]. The new pancreatic lesions found in postoperative follow-up can be distinguished into benign and malignant lesions; if warranting an indication for resection, the lesions are referred to in current guidelines as “clinically significant residual pancreatic lesions” [23].
The average cumulative incidence of clinically significant residual pancreatic lesions after 5 years is 10%, with the risk increasing after this period [23, 113–115]. Patients with HGD or a family history of pancreatic cancer are also at higher risk of developing metachronous pancreatic cancer, which explains the recommendation for more frequent follow-up (every 6 months) [23]. HGD is a marker of an aggressive IPMN phenotype that can spread throughout the pancreas [116], increasing the risk of new lesions requiring intervention. In such patients, multifocal lesions can develop independently of the originally removed lesion, further confirming the need for close surveillance [23].
For patients without additional risk factors, such as HGD or family history, the risk of tumor recurrence or progression is lower, allowing for less intensive follow-up – once every 12 months [23]. However, because of the long-term risk of clinically significant residual pancreatic lesions, which can increase even 5 years after surgery, surveillance should continue throughout the patient’s life unless surgery becomes contraindicated due to other clinical burdens [23].
To detect clinically significant residual pancreatic lesions, MRI, EUS, and/or contrast-enhanced CT examinations are recommended according to the proposed time schedule [23].
In patients undergoing total pancreatectomy due to a non-invasive malignant lesion, the specific surveillance associated with IPMN can be terminated if no abnormalities are observed in the 5-year postoperative period [23].
20. In the absence of indications for surgery, patients with pancreatic cystic tumors (MCN and IPMN) should be monitored with contrast-enhanced MRI or EUS. The first surveillance examination should be performed after 6 months, while the schedule of subsequent examinations depends on the dimensions and morphology of the lesion.
| Quality of evidence: low. |
| Recommendation strength: strong. |
| Total support: 59.1%, Support: 40.9%, Partial support: 0%, Partial objection: 0%, Objection: 0%, Total objection: 0%. |
Planning long-term surveillance of patients with IPMN without indications for surgery is important due to the risk of benign BD-IPMNs undergoing transformation to highly malignant tumors. Studies found that lesions smaller than 10 mm have a low risk of developing worrisome features (4.8% at 54 months) while larger lesions, especially those over 20 mm, are at a significantly higher risk of progression (48.8% at 23 months) [23].
The first follow-up examination should be performed after 6 months (contrast-enhanced MRI or EUS), the timing of subsequent examinations depending on the dimensions and morphology of the lesion. If the lesion shows no progression after the first surveillance examination, we suggest that lesions under 20 mm be examined every 18 months, while lesions over 20 mm be examined every 12 months. Cystic tumors above 30 mm in size should be subject to imaging evaluation every 6 months [23] – Figure 1.
The timing of surveillance discontinuation is a matter of controversy. Some authors suggest that small BD-IPMNs (< 20 mm) without worrisome features and stable for 5 years may not require further surveillance [117, 118]. However, other data suggest that the risk of pancreatic cancer being is independent of the size of the IPMN [119–121] and therefore recommend long-term surveillance, especially in older populations or patients with large numbers of small, asymptomatic lesions.
This approach is justified by the results of studies showing a persistently high risk of metachronous PDAC developing in patients with an IPMN-type lesion. Studies show that the cumulative 5-year risk of developing PDAC in this group of patients ranges from 2.2% to 3.0%, and can increase to 8.7–8.8% after 10 years [121, 122]. These data suggest that the surveillance period should be longer than 5 years, especially in high-risk patient groups [22, 66].
It should be emphasized that the surveillance of IPMN patients with no indications for surgery should not only involve the analysis of images obtained using the suggested modalities (MRI, EUS), but also take into account the overall clinical assessment and regular measurements of serum CA 19-9 levels [21, 22, 66]. The presence of worrisome clinical or morphological features related to the observed lesion or the onset of a new suspicious pancreatic lesion should always prompt a re-examination of the indications for surgery. It should be noted that although IPMN and MCN are distinct disease entities, differentiation between the two may pose some difficulty. The European and American College of Gastroenterology (ACG) guidelines suggest similar surveillance regimens for IPMN- and MCN-type lesions not subject to surgical resection [11, 21]. The diagnostic and therapeutic algorithm for suspected cases of IPMN or MCN is shown in Figure 1.
21. Asymptomatic patients with confirmed SCN-like cystic tumors should be followed up for 1 year. After this time, a follow-up based on verification of possible symptoms is recommended.
| Quality of evidence: low. |
| Recommendation strength: strong. |
| Total support: 40.9%, Support: 50.0%, Partial support: 4.5%, Partial objection: 4.5%, Objection: 0%, Total objection: 0%. |
Serous cystic neoplasms of the pancreas (SCN) are most common (60–75% of cases) in women, usually aged between 50 and 70 years [123, 124]. A study involving 2,622 SCN patients revealed that 61% of patients in the study group were asymptomatic at the time of diagnosis. Among those presenting with symptoms, nonspecific abdominal pain was the most common presentation, observed in 27% of subjects; 9% presented with pancreatic or biliary tract-related symptoms; 5% had diabetes, while 4% presented with other predominant symptoms, such as an abdominal mass, fatigue, nausea and vomiting [123].
SCNs can be distinguished from pancreatic cysts on the basis of certain features. For example, SCNs do not communicate with the pancreatic duct, in contrast to IPMNs. SCNs also present with four distinct morphological patterns: microcystic, macrocystic, mixed microcystic/macrocystic, and solid [124]. The most common (occurring in about 50% of cases) is the microcystic pattern. Microcystic SCNs are composed of small cysts less than 2 cm in size, separated by thin septa. They may also present with central fibrotic scars and calcifications, as observed in 30% of microcystic SCNs, giving them an overall “sunburst” appearance. Microcystic SCNs can also present with a honeycomb-like appearance, indicating a structure composed of numerous small cysts [124].
Macrocystic SCNs consist of multi-chambered cysts sized > 2 cm, separated by thin septa, without central scarring. This morphology accounts for about 30% of all SCNs [124–126]. Lesions with this structural pattern are more frequently located in the head of the pancreas and may cause jaundice [127]. Notably, macrocystic SCNs may also pose a significant difficulty in differential diagnosis. A study showed that 31% of macrocystic SCNs were misidentified as IPMNs, pancreatic neuroendocrine tumors (PanNETs), MCNs, pseudotumors, or ductal adenocarcinomas [128].
Given the limitations of independently performed abdominal CT and MRI scans, it appears that combining these two imaging modalities may provide greater accuracy in the evaluation of SCNs [124]. Quite frequently, however, CT and MRI scans fail to clearly identify the character of the cystic lesion, and expanded diagnostics are necessary. EUS-guided fine-needle aspiration (FNA) is a valuable diagnostic tool in differentiating pancreatic cysts, demonstrating sensitivity of 97% and specificity of 100% for determination of the cyst type, thus supporting proper therapeutic decision-making [129].
The dimensions of SCN-type tumors remain stable in about 60% of cases. Tumor enlargement is observed in 40% of patients with SCN-type tumors; however, the progression of lesions is slow, and new clinical manifestations are sporadic [21, 123, 124, 130, 131]. SCN is a benign lesion, and no cases of death attributable to malignant transformation of this type of cystic tumor have been reported. The reported SCN-related mortality rate is close to zero, with cases reported as “malignant” not meeting the WHO criteria for SCN [21, 123, 132]. Surveillance of SCN patients is required only when the diagnosis is uncertain. In these cases, a surveillance scheme analogous to that followed in the case of IPMN is proposed [23]. Asymptomatic patients with unambiguously confirmed SCN-like cystic tumors should be followed up for 1 year. After this time, it is recommended that follow-up examinations be resumed when necessary to verify any new clinical signs that may appear [23, 11].
Resection surgery for SCN is recommended only in patients with symptoms related to the cystic tumor compressing the adjacent organs and structures (i.e., bile duct, stomach, duodenum, and portal vein) [21].
22. In the case of comorbidities preventing surgical or systemic treatment, or in the case of the patient’s non-consent regarding such treatment, the surveillance of a pancreatic cyst is not reasonable.
| Quality of evidence: low. |
| Recommendation strength: strong. |
| Total support: 59.1%, Support: 36.4%, Partial support: 4.5%, Partial objection: 0%, Objection: 0%, Total objection: 0%. |
The decision to surveil a pancreatic cystic tumor should be preceded by the assessment of the risk of pancreatic cancer, with the patient’s comorbidities and life expectancy, as well as the patient’s eligibility for surgery, being taken into account [11, 21, 22, 66, 95]. The location of the cystic lesion to be surveilled is also important, as the risk of complications associated with distal pancreatectomy is usually lower than that associated with pancreatoduodenectomy [11, 21]. The decision to continue or discontinue surveillance, especially in older patients, should be based on the patient’s overall health and the patient’s preference [11, 23].
23. The risk of extrapancreatic cancers in patients with pancreatic cystic tumors is similar to that in the general population.
| Quality of evidence: very low. |
| Recommendation strength: weak. |
| Total support: 40.9%, Support: 50.0%, Partial support: 4.5%, Partial objection: 4.5%, Objection; 0%, Total objection: 0%. |
Despite previous reports suggesting an increased risk of extrapancreatic neoplasms in patients with pancreatic cystic tumors [133–135], most studies fail to unambiguously confirm such an association [136–139]. The analysis of current data indicates that the risk of extrapancreatic cancers in these patients is comparable to that in the general population [21, 23, 66]. The co-occurrence of extrapancreatic cancers may be related to other risk factors, such as the age of patients.
Currently, no separate recommendations are available with regard to the screening for extrapancreatic cancers in patients with IPMNs. However, isolated reports may indicate a higher incidence of gastrointestinal cancers (such as colorectal cancer or gastric cancer, among others) in these patients [140]. Therefore, once IPMN is diagnosed, an individual’s risk of extrapancreatic neoplasms should be assessed on the basis of other risk factors and their prevalence in a specific population [66]. Such management should determine further diagnostic measures and patient monitoring.
24. A pancreatic cyst with no worrisome features or high-risk stigmata is not a contraindication to organ transplantation.
| Quality of evidence: low. |
| Recommendation strength: strong. |
| Total support: 54.5%, Support: 45.5%, Partial support: 0%, Partial objection: 0%, Objection: 0%, Total objection: 0%. |
Patients undergoing preparation for transplantation should be subjected to a thorough evaluation of the cyst, especially if a mucinous lesion (IPMN/MCN) is suspected. In all cases, MRI scans and EUS-FNA are recommended to determine the possible presence of worrisome features or high-risk stigmata for HGD/IC. It is also recommended that laboratory tests be performed, including determination of serum CA 19-9 and glucose levels, as well as a detailed clinical evaluation of any cyst-related symptoms [141].
Short-term follow-up of patients with IPMN-BD following parenchymal organ transplantation did not reveal significant changes in cyst characteristics [142]. This suggests that even with post-transplant immunosuppression, IPMN-BD can be monitored in accordance with the standard surveillance regimen established for this type of lesion.
Summary
These guidelines, based on an analysis of the available literature and expert opinions, aim to provide a practical approach to the diagnosis, monitoring, and treatment of pancreatic cystic tumors. Due to the limited quality of available evidence, the recommended algorithms serve as a general framework and do not cover all possible clinical scenarios, underscoring the need to individualize the treatment on the basis of the patient’s specific characteristics and needs. We hope that this document will be helpful to clinicians involved in the diagnosis and treatment of pancreatic cystic lesions, providing up-to-date and practical support in the management of frequently complex cases.