Introduction
Colitis refers to inflammation of the mucosal lining of the colon. It is usually associated with mucoid or bloody stool, abdominal pain, tenesmus, fever and urgency. Chronic colitis in children comprises a heterogeneous group of disorders including chronic infections, allergic colitis, inflammatory bowel disease (IBD), colitis secondary to immune deficiency disorders or other rare causes such as Behcet’s disease, ischaemic colitis, or microscopic colitis [1].
Faecal calprotectin is a 36.5 kDa calcium and zinc binding protein which is mainly exhibited in the cytoplasm of the neutrophils [2]. It is excreted in the intestinal lumen in inflammatory conditions of the gut and can be used to distinguish inflammatory bowel conditions such as colitis from non-inflammatory causes. It indicates gastrointestinal inflammation. Faecal calprotectin is usually high in different types of colitis, but the degree of elevation is often related to the extent and severity of inflammation rather than the cause itself. It is a non-invasive initial test which may provide a clue to the anticipated type of colitis, guide the subsequent diagnostic steps and aid the paediatricians for early referral to gastroenterologists for patients presenting with chronic colitis [3, 4]. Moreover, the level of faecal calprotectin is a marker of mucosal healing and may reduce risk for endoscopic reassessment [5, 6].
Aim
The aim of this study was to assess the level of faecal calprotectin in different causes of chronic colitis, to measure the cut-off level to differentiate between IBD and non-IBD colitides and to study correlations of faecal calprotectin with different laboratory markers.
Material and methods
This work was conducted as a prospective study for 1 year, from June 2018 to the end of May 2019. The study included all patients aged 2 months up to 18 years who presented with symptoms suggestive of chronic colitis (mucoid or bloody diarrhoea for 14 days or more) attending the Gastroenterology Clinic at Alexandria University Children’s Hospital. Infants with necrotizing enterocolitis or Hirschsprung-related enterocolitis were excluded. Formal informed written consent was obtained from the parents or the caregivers of all children before inclusion in the study. The study was conducted in accordance with the ethical standards of the institutional review board and with the Declaration of Helsinki (ZU-IRB#: 00012098). The study protocol was approved by the ethical committee of the Faculty of Medicine, Alexandria University, Alexandria, Egypt.
Demographic data comprising age, gender and demographic area were recorded. The following laboratory investigations were conducted among all children: CBC, C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), serum albumin, routine stool analysis including examination for ova, parasites or worms and faecal calprotectin. A stool specimen was obtained prior to administration of laxatives used to prepare patients for colonoscopy. It was collected by each subject using a disposable plastic bucket-type device to avoid contact with toilet water and simplify laboratory sampling. In children with diapers, stools were collected directly from the bottom into a test tube to avoid water absorption by the diapers, which may increase faecal calprotectin level [7]. Samples were stored at –20°C and thawed at room temperature before testing. The faecal calprotectin levels were analysed by a commercially available ELISA assay (Calprest, Eurospital Spa, Trieste, Italy) with the manufacturer recommended cut-off levels [8].
Results of faecal calprotectin were compared to the age-specific cut-off reference levels, with a higher cut-off level for children during the first year of life (< 350 µg/g) than for children aged from 1–4 years (< 275 µg/g) or older children (< 50 µg/g) [9–11]. However, a level of 200–400 µg/g was considered mildly elevated for children below 4 years and a level above 400 µg/g was considered significantly elevated while above 4 years a level of 50–200 µg/g was considered mildly elevated while a level above 200 µg/g was considered significantly elevated [8].
The colonoscopic examination was performed for all children included in the study. Gross macroscopic findings and extent of the inflammation were recorded. All the biopsy specimens were fixed in neutral buffered formalin and they were processed and stained with haematoxylin and eosin. Histopathological findings were reported. Final diagnosis was reached based on endoscopic and histopathologic findings.
Statistical analysis
Data were fed to the computer using IBM SPSS software package version 20.0. Quantitative data were expressed as the mean ± SD and/or as median (range). Qualitative data were described using number and percent. Comparison between different groups regarding categorical variables was tested using the χ2 test. When more than 20% of the cells had an expected count less than 5, correction for χ2 was conducted using Monte Carlo correction. Quantitative data were described using median, minimum and maximum for non-normally distributed data. For non-normally distributed data, the Kruskal-Wallis test was used to compare between different groups and the Mann-Whitney test was used to compare between two groups. Spearman’s coefficient was used to correlate between quantitative variables. The cut-off level of faecal calprotectin was determined using a receiver operating characteristic (ROC) curve to differentiate IBD from non-IBD colitides; a level of significance of 0.05 was used, equal/below which the results considered to be statistically significant [12].
Results
The study included 110 patients. Fifty-eight (52.7%) patients of the whole group were male. Seventy-two (65.5%) patients were from rural areas. The most common causes for colitis in our cohort were allergic colitis (50.9%), followed by IBD (38.1%) and infectious colitis (6.3%). These three main causes compromised 95.4% of the whole group. Immunodeficiency related colitis (2 cases with chronic granulomatous disease related colitis and 1 case with IPEX (immune dysregulation, polyendocrinopathy, enteropathy, X-linked)), Behcet’s colitis and microscopic lymphocytic colitis accounted for 2.7%, 0.9% and 0.9% respectively. Allergic colitis was the most common cause of chronic colitis in children aged less than 4 years (80.3%) while IBD was the most common cause of chronic colitis in children aged more than 4 years (83.3%) (p < 0.001*).
The most common findings during laboratory evaluations were elevated CRP, hypoalbuminaemia followed by anaemia (50.9%, 47.3% and 45.5% respectively). Patients with IBD had more leukocytosis, thrombocytosis, anaemia and hypoalbuminaemia. Patients with infectious colitis had the highest frequency of elevated levels of CRP followed by patients with IBD. Eighty percent of the patients had an elevated faecal calprotectin level (16.4% had mild elevation, 63.6% had significant elevation). The majority of IBD patients had significant elevation (92.7%). The levels of faecal calprotectin were significantly higher in children with IBD (Table I). Patients with pancolitis had significantly higher faecal calprotectin levels than those with left sided colitis (p < 0.001*).
Table I
Comparison between the three main groups according to laboratory investigations
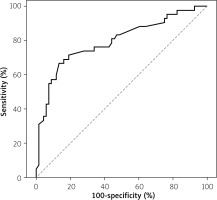
In the total group, faecal calprotectin was significantly correlated with the laboratory parameters CRP and ESR (Table II). The cut-off level of faecal calprotectin to differentiate IBD from non-IBD causes of colitis was 744 µg/g with 86.8% specificity and 66.7% sensitivity with the 95% confidence interval 0.703–0.885 (Figure 1).
Discussion
Chronic colitis is a major problem worldwide in different paediatric age groups. It has different presentations and aetiologies. An approach for differentiation between different types of chronic colitis is lacking. The epidemiology and management of this condition are different in the developing world [13]. Paediatricians often treat all cases with chronic colitis as infectious colitis, leading to delay in the diagnosis of other diseases.
Elevated inflammatory markers are associated with active colitis and usually correlate well with the severity of colitis, especially in IBD [14, 15]. In the present study, patients with IBD had the highest frequency of elevated inflammatory markers, especially leukocytosis and thrombocytosis. This corresponds to the laboratory abnormalities reported in multiple studies, which proved that elevated inflammatory markers have a diagnostic value in patients with IBD [14, 15].
Faecal calprotectin level is a sensitive, but not a disease-specific marker, to detect gastrointestinal inflammation and so can be used to distinguish colitis from other causes of non-inflammatory diarrhoea. It may also be used in primary care to aid decision making for early referrals to gastroenterologists [4]. The presenting symptoms of IBD and other causes of chronic colitis can be similar. Distinguishing them by clinical signs and symptoms can be sometimes difficult. Higher levels are usually reported in IBD than other causes of chronic inflammatory diarrhoea, which may help the gastroenterologist to distinguish IBD from other less urgent causes [4, 16]. This may help to prioritize patients with higher possibilities of IBD for earlier endoscopy especially in busy centres or in countries with limited resources. Similarly, in our study, we observed significant elevation of faecal calprotectin in IBD cases compared to other non-IBD causes. We also found that a cut-off level of 744 µg/g for faecal calprotectin may differentiate IBD from non-IBD cases with 86.8% specificity and 66.7% sensitivity. A cut-off level to distinguish between IBD and non-IBD is still lacking in the literature. A meta-analysis (9 studies, describing 853 patients) demonstrated that faecal calprotectin has a high overall sensitivity of 0.97 (95% confidence interval: 0.92–0.99) and a specificity of 0.70 (0.59–0.79) for diagnosing IBD [17].
In our study, the level of faecal calprotectin was significantly correlated with CRP and ESR. In a study in India, Samant et al. reported that faecal calprotectin correlates well with CRP but not ESR in patients with ulcerative colitis [18]. Another study reported the correlation of faecal calprotectin to CRP in Crohn’s patients, ulcerative colitis and IBD in total [19]. The literature is lacking studies about correlations of faecal calprotectin with CRP and ESR in chronic colitis with all its different types. These correlations emphasize the value of faecal calprotectin in assessing severity of IBD and possibly other causes of non-IBD colitides.
Data about faecal calprotectin level and cut-off levels are lacking. Few studies have compared faecal calprotectin level in IBD versus non-IBD as causes of chronic colitis. Moreover, few studies have correlated faecal calprotectin with other laboratory markers. This work highlighted the value of the level of faecal calprotectin in predicting the cause of chronic colitis even before definitive diagnosis by endoscopic and histopathological assessment and so early referral to the gastroenterologist.