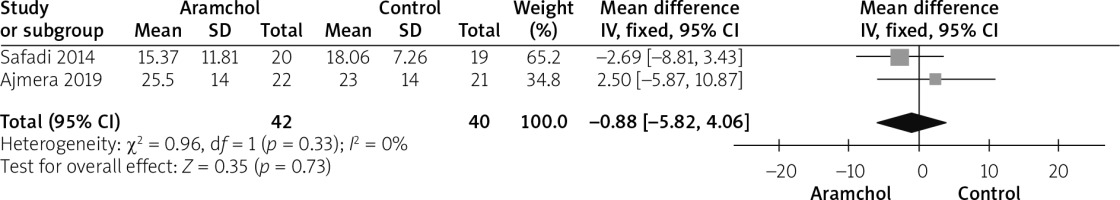Introduction
Nonalcoholic fatty liver disease (NAFLD) comprises a wide range of related liver disorders affecting mainly people who drink no or very little alcohol [1, 2]. Recently, it has become the most common cause of chronic liver disease, especially in the obese population of the western world, with a potential further increase in prevalence in the coming years [3–6].
Histologically it shows different stages from simple fat accumulation to liver cirrhosis. The earliest stage of the disease comprises a process of excess fat accumulation, called simple fatty liver or steatosis. The deposited triglycerides form cytoplasmic droplets in hepatocytes. Steatosis is often a self-limited process but may progress to a more advanced stage called non-alcoholic steatohepatitis (NASH). NAFLD occurs mainly in the obese, in whom insulin resistance occurs. No one knows the exact pathogenesis, only the limited treatment options [2, 7, 8].
The main pathophysiology of NAFLD is increased free fatty acid (FFA) delivery to the liver, as adipose tissue shows increased insulin resistance [9, 10]. Also, hyperinsulinaemia and excess carbohydrate intake may lead to an increase of de-novo lipogenesis, which represents a distinct character of NAFLD [11]. Despite the high prevalence of NAFLD, it shows no symptoms and is diagnosed incidentally during follow-up for other medical conditions [8]. Since obesity represents the main risk factor, physicians directed the different management plans towards lifestyle modification and weight reduction. Weight loss shows an improvement of both predisposing factors and liver histology in patients with NAFLD [12]. In addition, bariatric surgery represents an option to treat morbidly obese patients with NAFLD, but there are insufficient data to make it a valid choice [13].
Hence, the need for pharmaceutical treatment has increased, to prevent further development of NAFLD. The use of pharmaceutics mainly tends to target the management of associated diseases with NAFLD, including high blood pressure, blood glucose, and cholesterol levels. The progress in understanding the pathogenesis of NAFLD helps in developing specific, targeted medications.
Aramchol (arachidyl amido cholanoic acid) represents a new synthetic molecule. It results from the conjugation of 2 components: cholic acid (bile acid) and arachidic acid (saturated fatty acid). It inhibits stearoyl coenzyme A desaturase (SCD1). SCD1 represents the key enzyme of fatty acid metabolism in the liver. This leads to a direct anti-steatosis effect by decreasing fat synthesis and increasing fat oxidation, which results in the reduction of liver fat storage [14, 15]. Many studies found that Aramchol significantly reduced the fat content of the liver in animals with a high-fat diet, improved insulin resistance, and reduce the atherogenic effect [16–19]. Since the discovery of its effect on animals, different clinical trials have investigated its efficacy and safety on liver fat reduction. A clinical trial on NAFLD patients found that a specific dose of Aramchol (300 mg) for 3 months reduced the fat content of the liver by nearly 12.5% compared to placebo, with no serious drug adverse events [20]. This makes Aramchol a promising drug in the treatment of NAFLD. Recent reviews have discussed the available pharmaceutical options for NAFLD in general, with a lack of systematic reviews on Aramchol. This makes it difficult to formulate comprehensive evidence for the clinical use of Aramchol in NAFLD.
Aim
Hence, in this systematic review and meta-analysis, we aim to evaluate Aramchol in NAFLD patients, regarding its efficacy according to different randomized clinical trials.
Material and methods
In our systematic review and meta-analysis, we followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [21]. All steps of this study were performed according to Cochrane’s handbook of systematic reviews of interventions [22].
Literature search
We searched four databases: PubMed, SCOPUS, Cochrane CENTRAL, and Web of Science, without any restrictions on time or language. We performed our search using the following search strategy: (aramchol OR arachidyl amido cholanoic acid OR scd1 inhibitor) AND (hepat* OR steato*).
Eligibility criteria
Results from searching the literature were marked as included if they met the following eligibility criteria: (i) Population: patients with non-alcoholic fatty liver disease, (ii) Intervention: Aramchol with any route of administration. (iii) Comparator: any control. (iv) Outcomes: alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (AP), glyvated haemoglobin (HbA1c), total cholesterol (TC), triglyceride (TG), HOMA-IR, and insulin level. (v) Study design: only randomized clinical trials were included in our study (RCTs). Our exclusion criteria were as follows: (1) non-randomized controlled clinical trials, (2) studies that did not report data or measures for our selected outcomes, (3) single-armed trials, (4) those with no available full-text, or (5) animal studies.
Screening of results
After retrieving the search results, we exported the data into Endnote X8.0.1 (Build 1044), with the automatic removal of any duplicates. We screened the included articles manually through 2 steps: the first step was the title and abstract screening, and the second step was full-text screening. Two independent authors conducted the screening steps and obtained the full-text files for all included studies based on our criteria for eligibility criteria. A third author solved any discrepancies.
Data extraction and analysis
After the screening process was completed, we performed the data-extraction step. We extracted the data into 4 main categories: 1) baseline and demographic data of patients in each study, including age, body mass index, male/female ratio, and BMI. 2) Baseline values of ALT, AST, Alkaline phosphatase, HOMA-IR, and HbA1c. 3) Baseline values of total cholesterol, HDL, LDL, and insulin. 4) Data for analysis that consisted mainly of our included outcomes: ALT, AST, AP, HbA1c, TC, TG, HOMA-IR, and Insulin. We also extracted the data about the 7 domains assessing the risk of bias according to Cochrane’s risk of bias.
Statistical analysis
We performed our analysis using Review Manager Software (RevMan 5.4.1) under the inverse variance method. Continuous data were expressed using the mean difference (MD) and standard error, relative to 95% confidence interval (CI), while dichotomous outcomes were expressed using the percentage and total. Two main tests indicate inconsistency among studies [23]: the I2 test (I2) and the p-value of the χ2 test. The outcomes with I2 > 50%, p < 0.1 were considered heterogeneous, while outcomes with I2 < 50%, p > 0.1 were considered homogeneous, according to the Cochrane Handbook [22]. We performed the analysis of homogeneous data under a fixed-effects model, while heterogeneous data were analysed under the random-effects model.
Quality assessment
We performed the quality assessment of this meta-analysis by using the guidelines of the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE). We included only the controlled trials and excluded the observational evidence. To assess the risk of bias among the included studies, we used Cochrane’s risk of bias tool [24]. The tool depends on the following domains for assessment of the risk of bias: 1) proper randomization; 2) blinding allocation of the included patients into each group; 3) blinding of patients only (single-blinding), blinding of both personnel and participants (double-blinding), or not blinding at all; 4) attrition bias; 5) selection bias (outcomes reported matches with that of the protocol or not); 6) awareness of the outcome assessor (whether blinded or not); and 7) other bias. The total risk of bias for the studies was also assessed.
Results
Summary of included studies
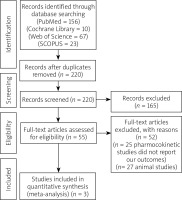
The results of our literature search are described in detail in the PRISMA flow diagram (Figure 1). We present the analysis of 231 patients with NAFLD from 3 studies [20, 25, 26]. A total of 143 patients were allocated to the Aramchol group, while 88 patients were allocated to the control group. The mean age of patients in the Aramchol group and control group was 46.33 years and 48.7 years, respectively. Table I shows a detailed summary of included patients and their demographic data and BMI.
Table I
A detailed summary of the included participants and their demographic data
Results of risk of bias assessment
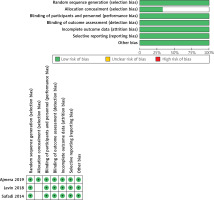
We found an overall low risk of bias, according to Cochrane’s tool [10]. All studies [20, 25, 26] were at low risk of bias regarding randomization, blinding of participants and personnel, attrition, and selective reporting. Concerning allocation concealment, Ajmera et al. [25] reported proper allocation concealment, so it was categorized as “low risk” of bias, while the other 2 studies [20, 26] did not report sufficient details about concealment, so they were put to unclear risk of bias. Regarding blinding of outcome assessment, 2 studies [25, 26] reported adequate blinding, so they were put to low risk of bias, while the other study did not report enough details regarding blinding of assessors, so it was categorized as “unclear risk”. A detailed illustration of the risk of bias of included trials is summarized in Figure 2.
Analysis of outcomes
Alanine aminotransferase
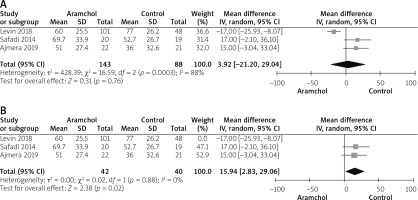
All studies [20, 25, 26] reported ALT outcome. The overall mean difference did not show any significant difference between both groups (MD = 3.92 [–21.20, 29.04], p = 0.76). Pooled analysis was heterogeneous; p = 0.03; I2 = 88%, as shown in Figure 3 A. We solved heterogeneity by excluding Levin et al. [26]; p = 0.88; I2 = 0%. The overall analysis after solving heterogeneity showed a significant increase in ALT levels in the Aramchol group (MD = 15.94 [2.83, 29.06], p = 0.02) as show in Figure 3 B.
Aspartate aminotransferase
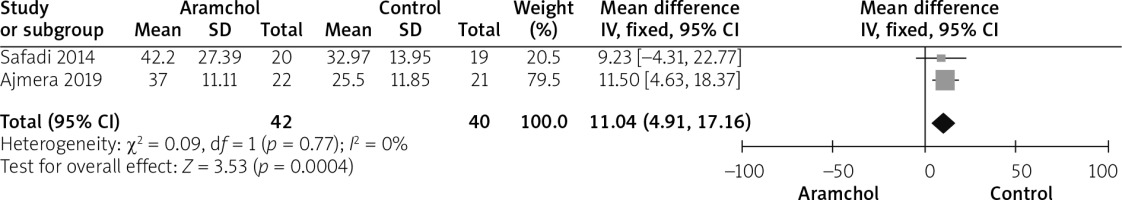
Two studies [20, 25] reported AST outcome. The AST level was significantly higher in the Aramchol group (MD = 11.04 [4.91, 17.16], p = 0.04). Data was homogeneous; p = 0.77; I2 = 0%, as shown in Figure 4.
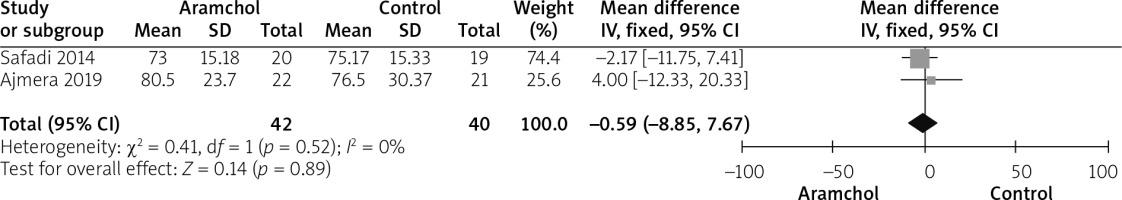
Alkaline phosphatase
Two studies [20, 25] reported the AP outcome. The overall mean difference did not show any difference between both groups (MD = –0.59 [–8.85, 7.67], p = 0.89). Data were homogeneous; p = 0.52; I2 = 0%, as shown in Figure 5.
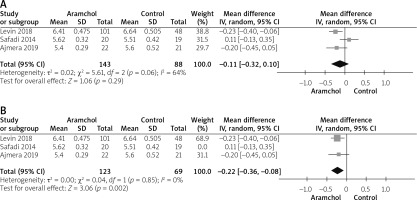
HbA1c
All studies [20, 25, 26] reported the HbA1c outcome. The combined analysis showed no difference between either group (MD = –0.11 [–0.32, 0.10], p = 0.29). Pooled analysis was heterogeneous; p = 0.06; I2 = 64%, as shown in Figure 6 A. We solved heterogeneity by leaving Safadi et al. [20] out; p = 0.85; I2 = 0%. The combined mean difference after solving heterogeneity favoured the Aramchol group (MD = –0.22 [–0.36, –0.08], p = 0.02), as shown in Figure 6 B.
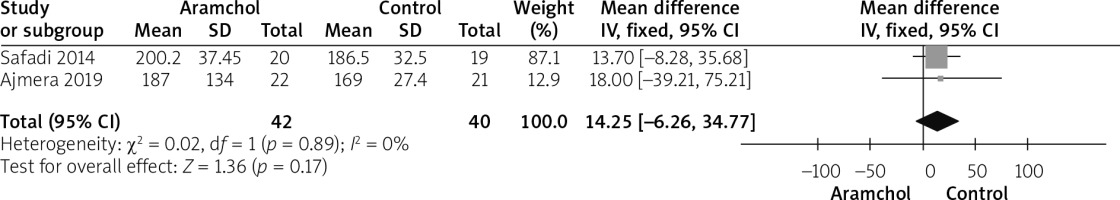
Total cholesterol
Two studies [20, 25] reported TC. The overall analysis did not show any significant difference between both groups (MD = 14.25 [–6.26, 34.77], p = 0.17). Pooled analysis was homogeneous; p = 0.89; I2 = 0%, as shown in Figure 7.
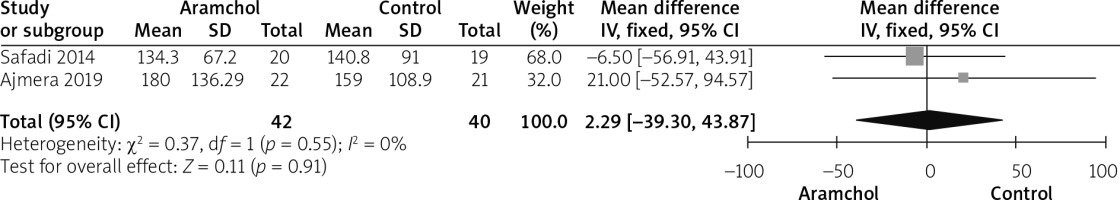
Triglyceride
Two studies [20, 25] reported triglyceride. Pooled analysis did not favour any group over the other (MD = 2.29 [–39.30, 43.87], p = 0.91). Data were homogenous; p = 0.55; I2 = 0%, as shown in Figure 8.
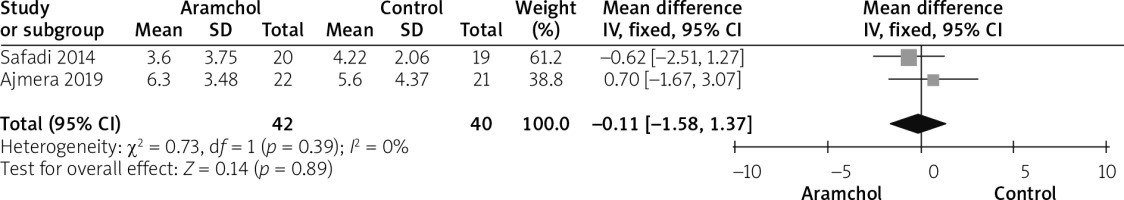
HOMA-IR
Two studies [20, 25] reported HOMA outcome. The overall analysis did not show any significant difference between both groups (MD = –0.11 [–1.58, 1.37], p = 0.89). Pooled analysis was homogeneous; p = 0.39; I2 = 0%, as shown in Figure 9.
Insulin levels
Insulin was reported by 2 studies [20, 25]. The overall mean difference did not show any significant difference between both groups (MD = –0.88 [–5.82, 4.06], p = 0.73). Data were homogeneous; p = 0.33; I2 = 0%, as shown in Figure 10 (Tables II, III).
Table II
Baseline values of ALT, AST, alkaline phosphatase, HOMA-IR, and HbA1c
Table III
Baseline values of total cholesterol, HDL, LDL, and insulin
Discussion
In this meta-analysis, we included 231 patients with NAFLD treated with Aramchol from 3 randomized controlled trials. We found that Aramchol did not show any significant reduction in AST and ALT levels. The results did not also show any significant difference between Aramchol and placebo regarding the level of plasma cholesterol, triglyceride, AP, HbA1c, and HOMA score.
ARRIVE [25] is the first randomized, double-blind, placebo-controlled trial that used an advanced MRI-based measure of body composition, including total visceral adipose tissue (VAT) and vibration-controlled transient elastography (VCTE) before and after the treatment. The trial found that Aramchol was not associated with a significant reduction in hepatic fat, aminotransferases, or liver stiffness compared with placebo. On the other hand, Aramchol may lead to a significant reduction in ALT level, especially among obese patients with HIV-associated NAFLD. Throughout the trial, Aramchol was safe and well-tolerated with no significant side effects and a similar safety profile to placebo. The ARRIVE trial did not include the histological examination of the liver as a diagnostic measure, which may, in turn, limit the ability to detect the early improvement in disease activity, especially those related to the improvement in ALT. Aramchol is a potent oral hepatic stearoyl-CoA desaturase 1 (SCD1) modulator. It belongs to medications targeting the primary metabolic process; specifically, the subgroup of inhibiting de-novo lipogenesis [27, 28]. SCD-1 is the rate-limiting enzyme in hepatic lipogenesis. Aramchol causes downregulation of SCD-1, which converts saturated fatty acids into monounsaturated fatty acids [29]. SCD-1 downregulation results in a reduction in hepatic lipogenesis, adiposity, liver triglycerides, and obesity resistance [30]. In addition, it acts on (ABCA1) transporter, a cholesterol efflux pump, producing an antiatherogenic effect [31].
In animal models, a trial by Iruarrizaga-Lejarreta et al. [19] found that Aramchol played a significant role in the improvement of steatohepatitis and fibrosis. This improvement was explained by the effect of the drug on SCD-1 and the use of an alternative trans-sulphuration pathway maintaining cellular redox homeostasis.
A trial by Safadi et al. [20] found that Aramchol was associated with a significant dose-dependent improvement in liver function. Patients treated with high-dose Aramchol had a significant reduction of symptoms related to liver cell failure compared with placebo. It was also found that daily administration of 300 mg Aramchol was tolerable and safe and causes a significant reduction in reduces liver fat content in patients with NAFLD. However, the short therapeutic protocol of the trial represented a strong limitation to estimate the clinical effect of Aramchol on liver fat.
In a 1-year ARREST study [26], Aramchol was found to reduce liver fat and improve the biochemical markers of the liver. The drug was also found to reduce liver fibrosis in a dose-response pattern. Administration of Aramchol 600 mg demonstrated higher rates of NASH resolution and fibrosis reduction with excellent safety outcomes. The major strengths of our analysis were that we included only clinical trials, which is important to ensure the strongest evidence according to GRADE. Another strength is that all the included studies were at low risk of bias in general. We tried to solve any inconsistency among studies using appropriate methodologies reported by Cochrane’s handbook [32].
The heterogeneity in some outcomes was the major limitation; however, we tried to solve the heterogeneity by subgroup analysis. Another limitation was the small sample size and a low number of published clinical trials; therefore, we still recommend more trials to combine Aramchol with other medications or to use higher doses below the safety margin of the drug.