Introduction
Inflammatory bowel disease (IBD), including ulcerative colitis (CU) and Crohn’s disease (CD), is an emerging epidemiological problem in terms of the burden it imposes on public health care. The last studies show that morbidity due to IBD is still increasing around the world [1, 2]. So also in Poland, the group of patients diagnosed with IBD is consistently growing and up to the most current studies, it is 100 thousand individuals [3]. This trend is associated with the growing number of patients who require the use of innovative biological treatment, which is still relatively expensive [4, 5]. Biological treatment in Poland is available within health insurance and is provided through therapeutic programs fully reimbursed by the National Health Fund (Narodowy Fundusz Zdrowia – NFZ), which is a national public payer. According to the terms of these programs, this publicly funded treatment is available only for those patients who have not responded to conventional treatment, including 5-aminosalicylic acid, steroids, thiopurines, or in which this kind of treatment is contraindicated [6, 7]. In Poland, the most widely used biologic drugs for the treatment of IBD are infliximab (IFX) and vedolizumab (VDZ). Infliximab is a chimeric IgG1 monoclonal antibody protein that contains murine and human components that inhibit tumor necrosis factor-α (TNF-α). TNF-α is a signalling protein involved in acute phase reaction and systemic inflammation. Infliximab stops the cascade of the inflammatory reaction by blocking the existing TNF-α on cell surfaces, including the membrane lining the bowel surface, leading to mucosal healing. Infliximab was approved by the FDA for the treatment of CD in 1998, and UC in 2005 [8]. Vedolizumab is a humanized IgG1 monoclonal antibody that binds specifically to the α4β7 integrin, which is preferentially expressed in gut-homing T helper lymphocytes. By binding to α4β7 on certain lymphocytes, VDZ inhibits the adhesion of these cells to the mucosal addressin cell adhesion molecule-1 (MAdCAM-1) and reduces gastrointestinal inflammation. Vedolizumab was approved by the FDA for the treatment of moderate to severe CD and moderate to severe UC in May 2014. In clinical practice, biological treatment with IFX and VDZ consists of an induction phase, which involves three doses administered at weeks 0, 2, and 6, followed by an evaluation of the response. In responders, maintenance treatment is administered. The evaluation of response to treatment is based on clinical symptoms, endoscopic evaluation, and determination of biochemical markers based on serum and faecal. During years of use of VDZ and IFX in the management of IBD, the effectiveness and safety of these agents have been demonstrated. Unfortunately, there is still a group of patients who demonstrate only limited efficiency of their use. The primary nonresponse refers to the situation in which the given drug remains ineffective within the induction phase. This affects approximately 30% of patients, and the reason remains unknown [9–11] and requires switch to the drug. On the other hand, secondary nonresponse is a situation in which a patient who initially responded to the induction phase loses drug efficiency during the maintenance phase of treatment. It is estimated that during the maintenance phase about 40% of primary responders become insensitive to biologic therapy [12–14]. In such cases of secondary efficiency loss, dose escalation by dose doubling or shortening the interval between drug administration is recommended for IFX. For VDZ, only shortening the intervals between doses is an option. To optimize the treatment of CD and UC with biologic agents, it is recommended to monitor the drug concentration in serum [15, 16].
Aim
The aim of the study was to evaluate the association of concentrations of biologic drugs: VDZ or IFX with the clinical condition of patients with IBD during therapy.
Material and methods
The study comprised 82 adult patients with CD and CU, treated with VDZ and IFX at the Maria Sklodowska-Curie National Research Institute of Oncology, Warsaw, Poland; Department of Gastroenterology. Thirty-six patients with CU were treated with VDZ, while IFX was administered to 46 patients with CD. The biologic drugs were reimbursed by the National Health Fund within the framework of so-called drug programs. Patients had severe to moderate CD (CDAI > 300) or UC (score > 6 total Mayo score) with an inadequate response to conventional therapy, including corticosteroids and thiopurines, or were intolerant to such treatment or had contraindications to it. Before proceeding with biological treatment, patients sign consent to participate in the program, which includes regular visits and blood tests to assess response and safety of treatment.
In induction therapy, both VDZ and IFX were administered every 6 weeks, with no change in dose, as indicated in the summary of product characteristics. If the response to VDZ treatment was worse, only the interval between doses was shortened, e.g. to 4 weeks. Both IFX and VDZ were administered intravenously at weeks 0, 2, 6 and then, in patients who responded, every 8 weeks. In CD, the response to IFX (CDAI) was evaluated at week 6 and was defined as remission as CDAI of 150 points. In UC, the response to VDZ was assessed using the total Mayo score at week 14. The clinical response to treatment was defined as a reduction in disease activity of at least 3 points in the Mayo score, and remission was disease activity of 0–1 points. However, the endoscopic response was defined as a reduction in the Mayo endoscopic score by at least 1 point, and endoscopic remission was defined as a score of 0–1 points. Blood samples were taken from all IBD patients at the control visit and before the administration of the next dose of the drug. Serum was stored at –20°C until testing. Serum drug concentrations were determined by ELISA with RIDASCREEN kits from R-Biopharm AG. The RIDASCREEN® IFX and VDZ monitoring assays use highly specific monoclonal antibodies (MA-IFX6B7, MA-VDZ6F3, and MA-VDZ6E6), which were isolated and characterized at KU Leuven.
Statistical analysis
The Statistica 9.0 (StatSoft) was used for statistical calculations. Nonparametric tests, such as the Mann-Whitney U test, the Kruskal-Wallis test, the Spearman rank correlation coefficient and the χ2 test, were used for statistical calculations. The diagnostic power of the parameters determined was analyzed using the MedCalc program. The receiver operating characteristic (ROC) analysis was applied to determine our own cut-off points for the parameters tested depending on the clinical condition. Pairwise nonparametric comparisons of the neighbouring AUCs were performed by the Wilcoxon signed-rank test.
Results
The study included 82 IBD patients treated with VDZ or IFX antibodies. The characteristics of the group patients (including gender, age) are shown in Table I. In the first stage of the study, we analyzed the serum concentration of VDZ after the first dose (induction phase) in a group of 36 patients with UC. In this group, after the 300 mg dose, the median serum drug concentration was 17.6 µg/ml. Analysis of the relationship between serum VDZ concentration and response to treatment (endoscopic remission and endoscopic response and clinical response) in these patients did not show statistical differences (p = 0.219) (Table I).
Table I
Clinical characteristics of patients with inflammatory bowel diseases; ulcerative colitis (UC), Crohn’s disease (CD), vedolizumab (VDZ), infliximab (IFX). *UC stage evaluated according to the total Mayo score, **CD stage evaluated according to the Crohn’s disease activity index (CDAI)
The highest median serum concentrations of VDZ were observed in patients with endoscopic remission or response compared to median concentrations in non-responders. Higher median VDZ concentrations were obtained in patients with an endoscopic response (18.4 µg/ml) compared to the median in patients without a response (15.3 µg/ml), although these differences were not statistically significant.
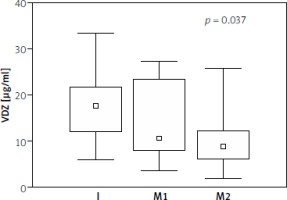
The association between serum VDZ concentrations and clinical response was not observed (Table II). The median serum VDZ concentration during the maintenance phase (after first and second maintenance doses) in patients with endoscopic remission remained at similar levels (12.3 µg/ml; 12.7 µg/ml), while its decrease was observed in patients without remission (10.1 µg/ml; 8.8 µg/ml). In patients without endoscopic remission, we obtained a significant decrease in the drug concentration in the maintenance phase compared to its concentration in the induction phase (p = 0.037) (Table III, Figure 1).
Table II
Serum concentrations of VDZ determined before the maintenance dose in patients depending on the type of response to treatment
Table III
Median serum VDZ concentrations in patients with UC during treatment. The Kruskal-Wallis test was used for statistical calculations
| Endoscopic remission | Serum collection | P-value | ||
|---|---|---|---|---|
| I | M1 | M2 | ||
| Yes | 18.2 µg/ml | 12.3 µg/ml | 12.7 µg/ml | 0.204 |
| No | 17.6 µg/ml | 10.1 µg/ml | 8.8 µg/ml | 0.037 |
Figure 1
Median (range) serum concentrations of VDZ in patients with UC without endoscopic remission, during treatment: I – induction dose, M1 – first maintenance dose, M2 – second maintenance dose. Spearman’s rank correlation coefficient test was used for statistical calculations (R = 0.37; p = 0.008)
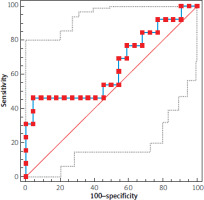
The receiver operating curve analysis was used to determine the VDZ cut-off value to differentiate patients with endoscopic remission after treatment from patients who did not respond to treatment (AUC = 0.629) (Figure 2). ROC analysis was also performed in the groups of patients with endoscopic response versus non-responders (AUC = 0.641).
Figure 2
ROC curves of VDZ in patients with UC without/with endoscopic remission. Non-parametric pairwise comparisons of the neighbouring AUCs were performed by the Wilcoxon test
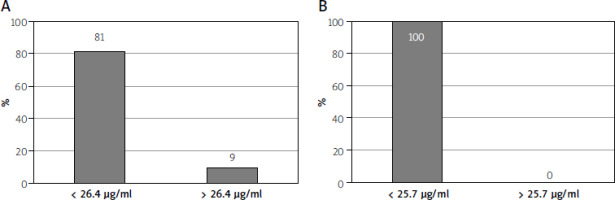
In the majority of patients, non-responders to treatment, serum VDZ concentrations below designated cut-off points were observed. Differences in the percentage of elevated scores were statistically confirmed by χ2 test: p = 0.002 for remission and p = 0.001 for endoscopic response (Figure 3).
Figure 3
Percentage of patients without endoscopic remission (A)/endoscopic response (B) according to the cut-off points determined for serum VDZ
In a group of 46 CD patients, up to 91% of the patients responded to treatment with IFX during the maintenance phase, according to the CDAI score. Median serum IFX concentrations in patients with remission were 7.4 µg/ml, while concentrations in 3 patients with mild and moderate activity were very low, 2.6; 0.09; 0.08 µg/ml respectively (Table IV). Patients who responded to treatment were qualified for further follow-up. Although 90% of patients still maintained remission, a decrease in median IFX concentrations was observed (4.8 µg/ml and 3.8 µg/ml) after the second and third maintenance doses. We did not analyse the serum IFX concentrations of CD patients for the induction phase, as only patients on maintenance treatment were included in our study.
Table IV
Response to treatment in patients with CD. Number of patients (%), concentrations of IFX (median and range) in patients depending on response to treatment; to evaluate disease activity, the Crohn’s disease activity index (CDAI) was used
Discussion
VDZ and IFX are biologic drugs registered for the treatment of IBD. For the therapeutic response the drug concentrations must be high enough. Hence, we conducted a study to evaluate the concentrations of biologic drugs during therapy, in relation to the clinical condition of patients with IBD. This meta-analysis result indicated that median trough VDZ concentrations were higher in patients with UC who achieved clinical remission or endoscopic remission [17]. Our study confirmed the association of VDZ concentrations with endoscopic remission or response to administered treatment in patients with UC, while a decrease in drug concentrations was associated with a lack of endoscopic remission or endoscopic response. We did not observe an association between serum VDZ concentrations and clinical response. The clinical response is a combination of three indicators: anatomical indicator, biological indicator, and assessment of the patient’s condition [18]. The last evaluation of the patient’s condition is a subjective opinion of the physician. However, in patients with endoscopic remission, the median serum VDZ concentrations after the first and second maintenance doses remained at similar levels, while in patients without remission we noted a significant decrease. The observed dynamics of changes in drug concentration may have prognostic implications, as our results showed that the higher serum VDZ concentration after the induction phase, the better response to treatment during the maintenance phase. The effectiveness of VDZ in achieving and maintaining remission was demonstrated by real-life observations in patients with IBD in Israel and Saudi Arabia. An Israeli study showed that the median concentration of VDZ at week 6 was higher in patients with clinical remission compared to the serum concentration in patients without response to treatment [19]. Observation in Saudi Arabian patients with a diagnosis of IBD indicates a positive effect of VDZ at week 14 of treatment. Furthermore, the clinical remission observed at week 14 was dependent on the median VDZ concentrations at week 6, and was significantly higher in responding patients compared to the concentrations in non-responders [20]. The efficacy of VDZ in the treatment of UC was evaluated in the GEMINI 1 clinical trial, which had a much longer follow-up period of 52 weeks. The results of a post-hoc analysis by Sandborn et al. [21] showed that patients receiving VDZ as maintenance therapy were more likely to achieve deep remission (mucosal healing) compared to placebo. Moreover, it was reflected in the symptomatic outcomes reported by the patients. Based on the ROC analysis, we determined cut-off points for VDZ concentrations that differentiate UC patients who achieved endoscopic remission or endoscopic response after treatment, however, we did not find statistical significance (AUC = 0.629; AUC = 0.641). The cut-off point for endoscopic remission was 26.4 µg/ml and for endoscopic response it was 25.7 µg/ml. A comparable AUC value (0.62) for endoscopic remission was obtained in the study by Vaughn et al. [22], while the cut-off point was 8 µg/ml, whereby the ROC analysis included the VDZ concentrations of patients with UC and CD and was determined in four different laboratories using different reagent kits. Some retrospective studies comprising two cohorts of patients with UC showed that 1 year of VDZ therapy is superior to 1 year of IFX therapy in terms of the frequency of achieving clinical remission [23]. Due to the small size of the group of patients with UC treated with IFX, we had not performed a comparative analysis in a shorter follow-up period. However, our study of IFX concentrations in 42 CD patients showed remission in up to 91% (according to the CDAI score) in the maintenance phase. The median serum antibody concentrations in these patients were the highest, whereas IFX concentrations in patients with mild to moderate disease activity were very low. The high percentage of patients responding to IFX treatment was also observed by Hibi et al. [24], where 48 of 57 patients showed a clinical response at week 14. The long-term outcome of the IFX treatment was obtained in real-life studies conducted by Schnitzler et al. [25]. These studies showed that 89.1% of patients with CD had improved clinical status after starting treatment and more than half of the patients treated with IFX in this cohort showed sustained clinical benefit at follow-up of approximately 5 years. Bortlik et al. [26] indicated a positive association of antibody trough concentrations in the maintenance phase over a 2-year follow-up period, where clinical response was observed in more than half of the patients. A study by Ward et al. [27] showed significant differences in median IFX concentrations between patients with and without biochemical remission and calprotectin normalization, whereby IFX concentrations in patients with calprotectin normalization were significantly higher than those observed in biochemical and clinical remission. Although serum IFX trough levels are thought to be associated with sustained efficacy during maintenance therapy, some data from the literature suggested that there was no relationship between serum drug levels and disease activity. Based on the observations of Gomes et al. [28] of 40 CD patients in Brazil, serum IFX levels in patients with and without remission were maintained at similar levels. Failure in monoclonal antibodies treatment can be caused by a variety of factors, including immunological mechanisms, related to the formation of antibodies against biologic drugs and their pharmacokinetics. Data from the literature indicated that immunogenicity with VDZ is low and anti-VDZ antibodies were observed in only a minority of patients [17]. Hence, it seems that the rates of immune-mediated pharmacokinetic failure may be low due to antibody formation; the situation is different for patients treated with IFX, where analysis of IFX concentrations was required in conjunction with analysis of anti-IFX antibody concentrations in the absence of therapeutic effects [26]. A different example is the removal of IFX as part of collopathy with protein loss, resulting in low trough concentrations of this antibody under active inflammation conditions [29]. Many observations to validate the efficacy of biologic drugs, e.g., VDZ and IFX, in patients with IBD showed that the lack of therapeutic effect in treatment and the associated decrease in serum drug concentrations should be analysed in relation to the other factors, including the determination of inflammatory biomarker levels [30], patient’s Body Mass Index, or history of steroid treatment. However, the evaluation of biochemical markers in patients with IBD is a routine test and an indirect method of assessing disease activity, while measuring serum drug concentrations seems to be a direct method to determine the chances of obtaining endoscopic or clinical remission.
In summary, regular measurement of serum drug levels during the induction phase and the maintenance phase can be a useful monitoring tool in the treatment of IBD. These results are pilot studies and the patients enrolled in the observation were a heterogeneous group, including patients who were treated with steroids. Therefore, the presented study corresponds to a real-life study, where the group of patients was not selected and drugs were administered to all patients who needed it, and those observations showed what the drug’s efficacy looked like in daily clinical practice.
Our study requires larger study groups and a longer follow-up. Increasing the group size would allow us to confirm the statistical significance of the results. Therapeutic drug monitoring (TDM) could help identify undertreated patients and adjust the optimal individualized dose for each of them. Our results have shown an important role in the determination of serum VDZ and IFX concentrations in clinical practice of treatment patients with IBD.
Conclusions
There is a relationship between the decrease in serum drug concentration and the absence of an endoscopic response. In our study, we determined the cut-off points (26.4 µg/ml or 25.7 µg/ml) for VDZ concentrations to differentiate patients with UC who have achieved remission or endoscopic response.
The sustained serum levels of VDZ concentrations in IBD patients determined in the maintenance phase confirm a positive response to this therapy, and a decrease in concentrations is associated with a decrease in response, which in practice may be important in optimising the dosage of biological treatment.










