Introduction
Acute pancreatitis is the leading cause of hospitalization due to gastrointestinal diseases in the United States [1]. The incidence of acute pancreatitis is increasing by about 5% per year in Europe and the USA [2–4]. Acute pancreatitis is an inflammatory disorder in which the pancreas causes an increase in cytokine release as a first phase of the disease and subsequent systemic inflammatory response syndrome (SIRS) [5]. Gallstones and alcohol abuse are the main causes of acute pancreatitis in the UK. About 25% of acute pancreatitis cases are attributed to alcohol abuse [6]. Severe acute pancreatitis is associated with high morbidity and mortality rates, reaching about 30% in cases of severe acute pancreatitis, while the overall mortality rate in acute pancreatitis is 5% [6]. The mortality rate is mostly attributed to complications and infection of pancreatic necrotic tissue [3]. The most common complications of acute pancreatitis include peri-pancreatic fluid collection, pancreatic pseudocyst, acute pancreatic necrosis, multiple organ failure, and abscess [7–11]. The management of acute pancreatitis depends mainly on the severity of the disease and whether it is associated with complications or not [12]. Early antibiotic treatment is associated with a significant improvement of necrotizing acute pancreatitis [13]. Previous studies reported that probiotics administration is associated with the reduction of complications and improvement of condition [14].
Probiotics are a mixture of living micro-organisms, which have beneficial effects on the host [15]. They improve the properties of the intestinal microflora [16]. This intestinal microflora aids the host in producing vitamins, degrading bile acids, and eliminating carcinogenic substances [17]. Prebiotics (symbiotics) are non-digestible fibres that help in gut integrity. Administration of prebiotics together with probiotics can improve their activity [18].
Bacterial translocation acts as a pathway by which the infection may reach the pancreatic necrosis [19]. Disturbed mucosal barrier and bacterial overgrowth are factors that help bacterial translocation [20]. Probiotics reduce the bacterial translocation in acute pancreatitis through beneficial effects on the immune system and the intestinal lumen. Probiotics also decrease the expression of pro-inflammatory cytokines such as IL6 and CRP. This action is mediated through toll-like receptors [21].
Material and methods
This meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [22] and the guidelines reported in the Cochrane Handbook for Systematic Reviews of Interventions [23].
Literature search
We searched 4 databases: SCOPUS, Web of Science, PubMed, and Cochrane CENTRAL, from inception until December 2020. We followed this search strategy with no restriction on time: (prebiotic OR probiotic OR symbiotic OR lactobacillus) AND (pancreatitis OR pancreatitides).
Eligibility criteria
We included all the studies that have the following criteria: (I) population: patients with acute pancreatitis, (ii) intervention: probiotic regardless of the dose and the mode of administration, (iii) comparator: placebo, or enteral feeding, (iv) outcomes: death, multiorgan failure (MOF), infected pancreatic necrosis, and SIRS as primary outcomes. The secondary outcomes were CRP (mg/l), APACHE II score, hospital stay (days), and ICU stay (days), (v) study design: we included only randomized clinical trials (RCTs). Our exclusion criteria were: (1) non-randomized controlled clinical trials, (2) studies that did not report data or measures for our selected outcomes, (3) single-armed trials, or (4) those with no available full text.
Screening of results
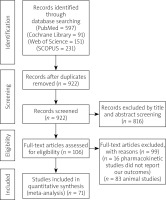
We exported the results of the search into Endnote X8.0.1 (Build 1044), with the removal of duplicates automatically by computer. After that, the studies were screened manually in 2 steps: first, title and abstract screening, then full-text screening for the preliminary included studies in the first step. Two independent authors performed the screening steps and obtained the full-text files for all included studies based on our eligibility criteria. A third author solved any deflection.
Data extraction and analysis
After the screening step, we extracted the data from the selected studies and categorized the data into 2 main groups: 1) baseline and demographic data of patients in each study including age, sample size, sex, alcoholism, APACHE II score, CRP level, Imrie score, and mean duration of symptoms before admission. 2) Data for analysis including outcome values of death, multiorgan failure (MOF), infected pancreatic necrosis, SIRS, CRP (mg/l), APACHE II score, hospital stay (days), and ICU stay (days). In addition to the previous 3 categories, we extracted data about the 7 domains assessing the risk of bias according to Cochrane’s risk of bias [24].
Data analysis
We used Review Manager Software (RevMan 5.4.1) to perform our analysis implementing the inverse variance method. We expressed dichotomous outcomes using the percentage and total, while continuous outcomes were expressed using mean difference (MD) and standard deviations (SD), relative to the 95% confidence interval (CI). Two main tests were used to indicate inconsistency among studies [25]: the I-square test I2 and the p-value of the χ2 test. The outcomes with I2 > 50%, p < 0.1 were considered heterogeneous, while outcomes with I2 < 50%, p > 0.1 were considered homogeneous, according to the Cochrane Handbook. Homogenous data were analysed using a fixed-effects model, while heterogeneous outcomes were analysed using a random-effects model.
Quality assessment
We evaluated the quality of this systematic review and meta-analysis using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) guidelines. We included only the controlled trials and excluded the observational evidence. According to the Cochrane risk of bias (ROB) tool for clinical trials, we performed the risk of bias (ROB) for the included studies. The tool depends on the following domains for assessment of the risk of bias: 1) proper randomization, 2) blinding allocation of the included patients into each group, 3) blinding of patients only (single-blinding), blinding of both personnel and participants (double-blinding), or not blinding at all, 4) attrition bias, 5) selection bias (outcomes reported matches with that of the protocol or not), 6) awareness of the outcome assessor (whether blinded or not), and 7) other bias. The total risk of bias for the studies was also assessed.
Results
Summary of included studies
We illustrated the results of the literature search in Figure 1. The analysis of 711 patients from 7 studies was performed [14, 26–31]. The patients received either probiotics or no treatment for the management of acute pancreatitis. A total of 359 patients were included in the probiotics group, while 352 patients were included in the control group. The mean age of patients in the probiotic group was 48.4 ±15 years, while that of the control group was 49 ±15.38 years. Table I shows a detailed summary of the baseline characteristics of the included studies and participants, the number of patients drinking alcohols, the mean duration of symptoms before admission (hours) (mean ± SD), and the APACHE II score (mean ± SD).
Table I
Data are reported as mean ± SD or n (%) unless otherwise specified
Results of risk of bias assessment
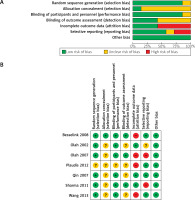
The result of the risk of bias assessment yielded an overall moderate risk of bias, according to Cochrane’s tool [24]. Regarding randomization, the majority of included studies reported randomization, so they were considered to be at low risk of bias [14, 26–28, 30, 31] except for the report from Plaudis et al. [29], which did not report sufficient data about randomization, Therefore, it was considered as unclear risk of bias. Concerning selection bias, Besselink et al. [26] reported adequate allocation concealment, while all the other included studies did not show adequate concealment [14, 27–31], so they were categorized as “unclear risk”. Participants and personnel were blinded in most of the included studies; therefore, they were considered as low risk of bias [14, 26–28, 30, 31] except for the study by Plaudis et al. [29], which did not report sufficient detail about blinding of participants and personnel, so it was categorized as “unclear risk”. Three studies reported adequate blinding of outcome assessment [26–28] and were thus categorised as low risk of bias, while the other 4 studies [14, 29–31] included insufficient data about the blinding of outcome assessment, so they were categorized as “unclear risk”. The remaining domains are illustrated in detail in Figure 2.
Analysis of outcomes
CRP (mg/l)
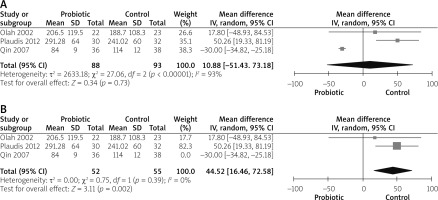
Three studies reported CRP [14, 27, 29]. The overall mean difference showed no significant difference between both groups (MD = 10.88 (–51.43, 73.18), p = 0.73). The data were heterogeneous (p < 0. 01, I2 = 93%), as shown in Figure 3 A. To solve heterogeneity we excluded the study by Qin et al. 2007 [30] (p = 0.39, I2 = 0%). The pooled analysis after solving heterogeneity showed that CRP was significantly decreased in the control group (MD = 44.52 (16.46, 72.58), p = 0.002), as shown in Figure 3 B.
APACHE II score
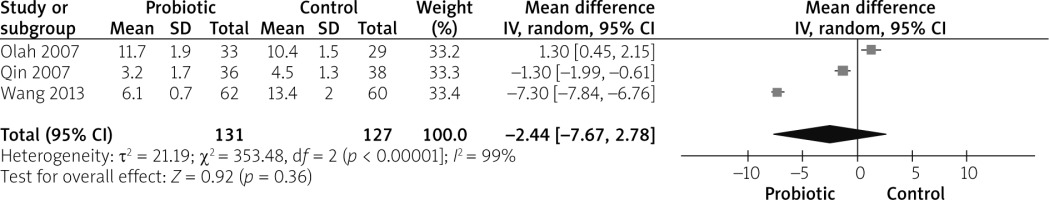
Three studies reported APACHE II score outcomes [27, 30, 31]. The overall mean difference did not show any significant difference between both groups (MD = –2.44 (–7.67, 2.78), p = 0.36). Pooled analysis were heterogeneous (p < 0.01, I2 = 99%), as shown in Figure 4. We could not solve heterogeneity by excluding one study or by subgroup analysis.
Hospital stay (days)
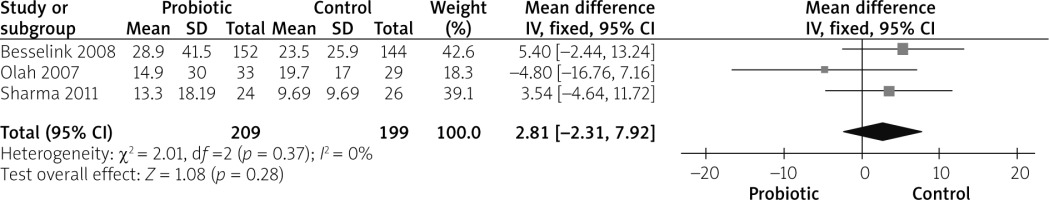
Duration of hospital stay was reported by 3 studies [26–28]. The combined mean difference showed no variation between both groups (MD = 2.81 (–2.31, 7.92), p = 0.28). The analysis was homogeneous (p = 0.37, I2 = 0%), as shown in Figure 5.
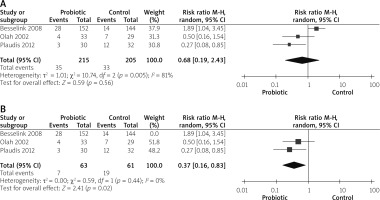
Death
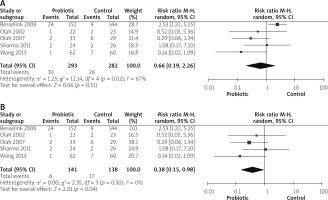
Mortality outcomes were reported by 5 studies [14, 26–28, 31]. No significant difference was observed between both groups (RR = 0.66 (0.19, 2.26), p = 0.51). Data were heterogeneous (p = 0.02, I2 = 67%), as shown in Figure 6 A. We solved heterogeneity by excluding the study by Besselink et al. 2008 [26] (p = 0.50, I2 = 0%). The pooled analysis after solving the heterogeneity favoured the probiotic group (RR = 0.38 (0.15, 0.98), p = 0.04), as shown in Figure 6 B.
Multiorgan failure (MOF)
MOF was reported by 6 studies [14, 26, 27, 29–31]. The overall risk ratio did not show any difference between both groups (RR = 0.60 (0.25, 1.44), p = 0.26). Analysis was heterogeneous (p = 0.07, I2 = 76%), as shown in Figure 7 A. We solved heterogeneity by excluding the study by Besselink et al. 2008 [26] (p = 0.51, I2 = 0%). The overall analysis after solving heterogeneity favoured the probiotics group (RR = 0.41 (0.25, 0.67), p = 0.04), as shown in Figure 7 B.
SIRS
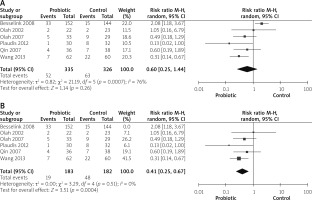
Three studies reported SIRS outcomes [14, 27, 30]. The overall risk ratio showed no significant variation between both groups (RR = 0.81 (0.29, 2.23), p = 0.68). Pooled analysis was heterogeneous (p = 0.04, I2 = 70%), as shown in Figure 8 A. Heterogeneity was solved by excluding the study by Olah et al. 2002 (p = 0.85, I2 = 0%). The pooled analysis after solving heterogeneity showed that use of probiotics decreases SIRS significantly (RR = 0.47 (0.23, 0.96), p = 0.04), as shown in Figure 8 B.
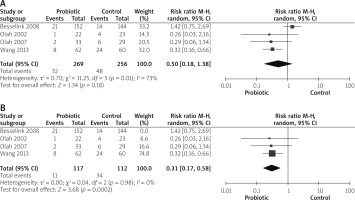
Infected pancreatic necrosis
Four studies reported infection pancreatic necrosis outcomes [14, 26, 27, 31]. The combined risk ratio showed no statistically significant difference between both groups (RR = 0.50 (0.18, 1.38), p = 0.18). Data were heterogeneous (p = 0.01, I2 = 73%), as shown in Figure 9 A. Heterogeneity was solved by exclusion of one study [26] (p = 0.98, I2 = 0%). The pooled analysis after exclusion favoured the probiotics group over the control group (RR = 0.31 (0.17, 0.58), p = 0.02), as shown in Figure 9 B.
Septicaemia
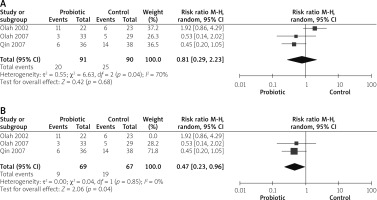
Five studies reported septicaemia outcomes [14, 26, 28–30]. The analysis did not show any significant variation between both groups (RR = 0.66 (0.29, 1.50), p = 0.32). Data were heterogeneous (p = 0.08, I2 = 51%), as shown in Figure 10 A. To solve heterogeneity, we excluded one study [26] (p = 0.72, I2 = 0%). Pooled analysis after solving heterogeneity favoured the probiotics group significantly (RR = 0.41 (0.19, 0.91), p = 0.03), as shown in Figure 10 B.
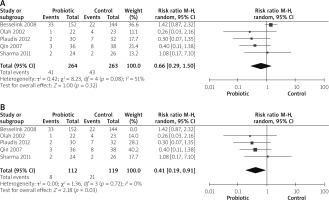
Need for operations
Three studies reported the need for operation outcomes [14, 26, 29]. The overall risk ratio did not show any significant difference between both groups (RR = 0.68 (0.19, 2.43), p = 0.56). Pooled analysis was heterogeneous (p = 0.005, I2 = 81%), as shown in Figure 11 A. After solving heterogeneity by excluding study Besselink et al. 2008 [26] (p = 0.44, I2 = 0%), the analysis favoured the probiotics group significantly (RR = 0.37 (0.16, 0.83), p = 0.02), as shown in Figure 11 B.
Discussion
In our meta-analysis, we found that there are no beneficial or harmful effects of probiotics over placebo concerning all important outcomes.
The large randomized “PROPATRIA” [26] trial was abandoned because of the harmful effects of probiotics in SAP. Sharma et al. [28] also reported that probiotics do not help in maintaining gut integrity or decreasing infectious complications because no change was observed in gut permeability and endotoxaemia. A meta-analysis by Gou et al. [32] showed the same results with significant heterogeneity. Conversely, Plaudis et al. [29], Qin et al. [30], Wang et al. [31], Oláh et al. [14], and Oláh et al. [27] showed that probiotics play a role in maintaining gut integrity and decreasing infectious complications.
As regards mortality, the analysis showed no difference between the probiotics and placebo in reducing the mortality rates with significant heterogeneity. The heterogeneity is attributed to the PROPATRIA trial. The types of probiotics may lead to different clinical outcomes, because different bacteria have variable adherence sites and different immunogenic effects. The probiotics used in the PROPATRIA trial, L. acidophilus and L. casei, (Ecologic 641; Winclove Bio Industries, Amsterdam, the Netherlands) were different from those used in the other included trials (Synbiotic 2000 Forte).
C-reactive protein reflects the inflammatory response in both groups. Plaudis et al. [29], and Oláh et al. [14] showed that the plasma CRP levels in the probiotic group demonstrate similar patterns to those in the control group, and probiotics may worsen the condition. However, Qin et al. [30] reported a significant reduction in CRP levels in the probiotic group.
Some strains of probiotics have anti-inflammatory effects because they reduce mucosal inflammation and modulate the cytokine levels [33]. They increase the glutathione (GSH) levels, which in turn scavenges superoxide and hydroxyl radicals, and decreases the interleukin-6 (IL-6) in adipocytes [34].
The APACHE II score is a multi-factorial scoring system that reflects the severity of different diseases. It is applied within 24 h of patient admission to the ICU [35]. Three trials: Qin et al. [30], Wang et al. [31], and Oláh et al. [27] demonstrated significant advantages of probiotics treatment in severe acute pancreatitis with a large reduction in infectious complications.
Regarding the hospital stay, Besselink et al. [26] and Sharma et al. [28] showed that there was no difference in the duration of hospital stay in the placebo and probiotic groups. However, Oláh et al. [27] reported a significant reduction in hospital stay.
Our meta-analysis estimates the effects of probiotics in patients with SAP. We only included randomized clinical trials, which ensures the highest evidence according to GRADE. All the included studies were at low risk of bias in general, which is another strength, and we conducted the analysis on a large sample size (711 patients). We solved the heterogeneity among studies using appropriate methodologies reported by Cochrane’s handbook [24].
The main limitation of our meta-analysis is the heterogeneity in some outcomes. However, we managed to elicit the attributing factors and overcome this inconsistency among the studies.