Introduction
Acute lymphoblastic leukemias (ALL) constitute about 35% of all childhood cancers. Although ALL is a heterogeneous group of diseases, it can be characterized by an abnormal proliferation and accumulation of immature cells – lymphoblasts in the bone marrow (BM), which can also spread to peripheral blood [1, 2]. In around 85% of childhood ALL cases, malignant transformation concerns immature B cells, resulting in B-cell precursor-ALL (BCP-ALL). Several somatic genetic alterations were described to trigger ALL, by modulating key regulatory processes, such as cell proliferation or responsiveness to apoptosis signals [1]. In about 75% of cases of pediatric BCP-ALL, different structural or numerical chromosome aberrations can be identified. Translocation t(12;21)(p13;q22), leading to the formation of fusion gene ETV6-RUNX1, and hyperdiploidy are the two most common and at the same time associated with the most favorable prognosis. In turn, rare aberrations such as t(9;22)/BCR-ABL1 and KMT2A gene rearrangements constitute adverse prognostic factors [3].
The last decade brought enormous advances in genomic research, which enabled more personalized, risk-directed therapy to be introduced for ALL patients. Specifically, this significantly improved the long-term survival and quality of life of pediatric ALL patients [4, 5]. Even though the statistics are encouraging, relapses among post-therapy ALL children still occur in about 20% of patients, implying that there is still much space for improvement [2]. The first step should be focused on early identification of genetic alterations holding the potential of direct introduction of the personalized therapeutic approach [4, 5]. Secondly, robust, standardized methods of monitoring the treatment efficiency should be introduced into routine practice. The latter can be achieved by assessment of minimal (or measurable) residual disease (MRD) i.e., quantification of residual leukemic cells at particular time points of the treatment protocol [5-8].
MRD detection is an already well-established, independent prognostic indicator in ALL and one of the key parameters in contemporary ALL treatment protocols used for patient stratification [5, 6, 8, 9]. Currently, two methods of MRD monitoring are employed: molecular technique based on clone-specific quantitative PCR amplification of immunoglobulin/T-cell receptor genes and flow cytometry (FC). The latter technique successfully competes with more time-consuming PCR-based techniques, and its application in a multiparametric (≥ 8-parameter) setting can provide the same sensitivity levels (down to 10–5) [10-12]. Advantages of FC include its speed, wide accessibility and affordability [12]. However, the predictive value of FC-MRD is affected by some technical challenges and interpretative complexities [6]. The most important is the lack, low number or instability of the so-called leukemia-associated immunophenotypes (LAIPs), which might concern 2% up to even 20% of pediatric ALL patients [13, 14]. This means that in this proportion of cases, the phenotype of leukemic blasts at diagnosis of BCP-ALL, determined using ‘classical’ markers (such as CD10, CD20, CD34, TdT, CD22, CD38, CD45), can be very similar to the phenotype of normal B-cell precursor cells (BCP, hematogones) emerging in BM in high amounts particularly at later follow-up time points. It is also evidenced that some antigens, e.g., CD10, CD20 and CD34, are prone to modulation (up- or downregulation) caused by different chemotherapeutic drugs [14, 15]. Thus, to prevent false negative MRD results it is crucial to determine strong LAIPs at diagnosis of BCP-ALL, e.g., by introduction of markers showing differential expression patterns from both leukemic blasts and all maturational stages of normal hematogones, and that at the same time are stable during the course of the therapy [15, 16]. The sensitivity of MRD assessment rises with the number of antibodies/antigens used to determine the LAIP of leukemic blasts [10]. In recent years, several studies were published in which potentially useful markers for MRD monitoring were assessed [14, 15, 17-23]. Among them, CD73 was shown as a promising marker for MRD monitoring in BCP-ALL [15].
CD73, also known as ecto-5’-nucleotidase, is an enzyme which catalyzes the conversion of 5-prime mononucleotides to nucleosides, mostly AMP to adenosine; thus overexpression of CD73 can be associated with high concentration of adenosine [24]. It has been proved that overproduction of extracellular adenosine promotes tumor growth and propagation by suppression of antitumor T-cell function [25]. Utilizing this knowledge, CD73 might be used in the future as a potent target in cancer immunotherapy [26].
The aim of the study was to determine and compare the expression of CD73 antigen on leukemic blasts at diagnosis and at days 15 and 33 of induction treatment in MRD-positive cases.
Material and methods
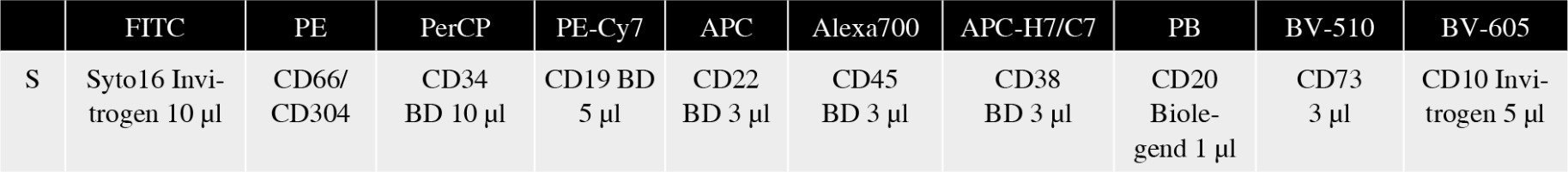
The study group consisted of 188 childhood BCP-ALL patients treated in 14 centers of the Polish Pediatric Leukemia and Lymphoma Study Group. Bone marrow samples collected at BCP-ALL diagnosis and days 15 and 33 of treatment were shipped to the reference center for FC-MRD detection – Medical University of Silesia in Katowice, Department of Pediatric Hematology and Oncology in Zabrze for central evaluation. The samples were processed in accordance with EuroFlow sample preparation protocols [27] including the EuroFlow bulk lysis protocol for samples collected at days 15 and 33 of treatment [28]. All samples at days 15 and 33 of treatment were stained with a ten-color monoclonal antibody panel and acquired with a BD FACS Canto flow cytometer (Fig. 1). For monitoring of BCP-ALL, EuroFlow recommends an 8-color panel combining CD304 and CD73 antigens together at the PE position. However, since 10-color stainings were available, these antigens were split apart, which enabled assessment of both single antigens in the current study.
Fig. 1
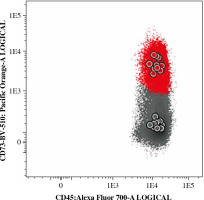
BCP-ALL MRD tube with CD73. Depending on the immunophenotype at diagnosis, CD66 or CD304 was used as a second additional marker in PE position
Since MFI values have usually large, fluorochrome-specific spread, often reaching high values, direct usage of MFI as a measure of antigen expression is difficult and not recommended. Thus, to determine antigen expression level, a normalized scale based on median fluorescence intensity (nMFI) was used [29]. As a positive reference for CD73 expression, a normal bone marrow CD73-positive mature B-cell subset was used, whereas a CD73-negative B-cell subset served as a negative reference population (Fig. 2). The range between mean MFI of CD73-positive and CD73-negative B-cell subsets was divided into 10 equal intervals: nMFI scores 0-1 corresponded to lack/low expression of CD73, while nMFI score of 10 represented maximal expression measured on the positive reference B-cell subset. MFI expression values on blast cells were compared to the nMFI scale and were assigned a score from 0 to 10. Since expression of CD73 on leukemic blasts could be abnormally high, the scale was extrapolated when necessary to assign nMFI scores higher than 10.
Fig. 2
Typical expression of CD73 on normal mature B-cells (red – CD73-positive subset, grey – CD73-negative subset). The MFI values of CD73 observed on those B-cell subsets were used as reference to create a normalized scale of CD73 expression in nMFI units
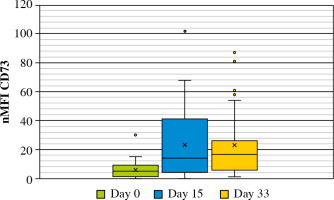
Expression levels of CD73 on leukemic cells were evaluated at diagnosis (day 0), at days 15 and 33 of induction treatment when routine FC-MRD evaluation was performed. Subsequently, expression levels in nMFI units were pairwise compared between day 0 and day 15 for every patient, and between day 0 and day 33 for patients who were still MRD-positive (31 patients). The modulation of CD73 expression was subsequently correlated with the occurrence of the most frequent genetic aberrations in BCP-ALL such as BCR-ABL1, KMT2A rearrangements, ETV6-RUNX1, TCF3-PBX1, IKZF1, as well as with hyper- and hypodiploidy.
For every patient, principal component analysis (PCA) was performed using Infinicyt (Cytognos, Salamanca, Spain) software. This approach determines the utility of individual markers contained in a single tube and grades them based on their individual contribution to separation of different cell populations. Thus, the use of PCA enabled us to compare the significance of CD73 in separation of blast cells from normal B cells vs. other well-established markers in the tube (e.g. CD10, CD20, CD38).
Results
CD73 expression at day 15 of induction treatment
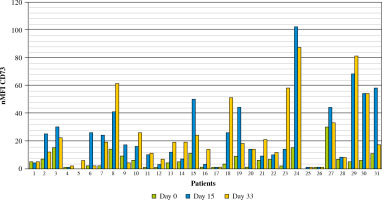
Out of 188 patients, 177 (94.1%) were MRD-positive at 15 day of treatment. Among MRD-positive patients, mean expression level of CD73 at diagnosis was 5 nMFI units, while at day 15 of treatment nMFI was 19. In 147 cases (83.1%) an increase of CD73 expression level at day15 of treatment was observed (mean increase of +17 nMFI units). In turn, CD73 expression level at day 15 was lower than at diagnosis in 13/177 (7.3%) cases (mean decrease of –3 nMFI units). In 17/177 (9.6%) cases the expression of CD73 remained at the same level (Figs. 3 and 4).
Fig. 3
Changes in CD73 expression at day 15 of treatment. nMFI values over the green line correspond to CD73 expression at diagnosis, whereas the blue line represents the nMFI values of CD73 for corresponding patients at day 15 of treatment. The results were ordered by increasing CD73 expression, from cases with decreased expression at day 15 (section I), through constant expression at day 15 (section II), to increased expression at day 15 (section III)
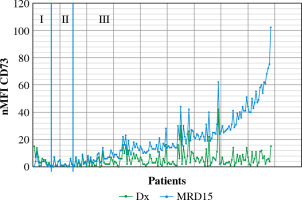
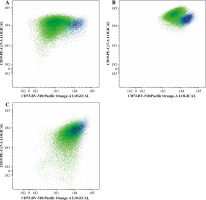
Fig. 4
Example of CD73 expression change in three patients. Green dots represents leukemic blasts at diagnosis, blue dots at day 15 of treatment
Interestingly, in 6 cases (3.4%) with lack of, or minimal CD73 expression at diagnosis (nMFI of 0-1) a significant increase at day 15 of induction treatment was observed (mean increase of +2 nMFI units). In contrast, only 3 CD73-positive cases (1.7%) had become CD73-negative at day 15 of induction treatment (Table 1).
Change of CD73 expression at day 33 of treatment
In 31 MRD-positive patients at day 33 of treatment, the mean nMFI value was 23 units (i.e., the same as at day 15 of treatment), as compared to mean nMFI of 6 at day 0 for these patients. An increase of CD73 expression was observed in 26/31 (83.9%) patients (mean increase of +17 nMFI units), while a decrease (by –5 nMFI) was observed only in 1 case. In 4 cases, the expression of CD73 showed no changes as compared to day 0. Interestingly, in 2 of these cases, the expression was very stable throughout all three time points (nMFI = 1) (Figs. 5 and 6).
Correlation of genetic data with CD73 expression changes
Correlation of the mean CD73 expression increase between day 0 and day 15 of treatment with the presence of BCR-ABL1, TCF3-PBX1, IKZF1 and ETV6-RUNX1 rearrangements did not reveal any significant differences as compared to patients without those aberrations (Table 2). In contrast, in patients with hyperdiploidy the increase of CD73 expression on blast cells at day 15 of treatment was significantly higher than in diploid patients (+19.7 vs. +12.1 nMFI units, respectively; p < 0.01, Table 2). Interestingly, in all 3 KMT2A-rearranged patients CD73 expression was very low at day 0 and did not increase during the early phases of treatment (nMFI of 0-1).
Table 2
Mean CD73 expression change in BCP-ALL patients of different genetic subtype
Significance of CD73 in blast cell separation
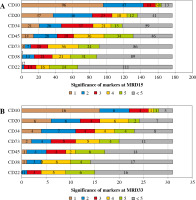
At day 15 of induction treatment in 97/177 (55%) cases, CD73 was within the top 5 markers contributing to separation of blasts from normal mature B cells. In 4/177 (2.2%) patients CD73 was the most significant antigen (Fig. 7A). CD73 showed greater utility than CD22 and CD38. At day 33 of induction treatment therapy CD73 was in the top 5 markers in 20/31 (65%) cases, being the most significant in 2 cases, which ranks CD73 before CD22, CD38 and CD45 (Fig. 7B). In one patient in which normal B-cell precursors were present together with leukemic blasts, CD73 was the second most significant marker separating these populations.
Discussion
Detection of MRD in BCP-ALL is one of the key elements in contemporary treatment protocols. FC-MRD level assessed at day 15 of induction treatment is one of the prognostic factors used for patient stratification and treatment effectiveness evaluation [30]. In the majority of cases, MRD level determination by FC is based on detection of LAIPs. However, it is known that some antigens contributing to LAIP (e.g. CD10, CD34 or CD20) can fluctuate during the treatment, which can potentially be problematic and lead to false negative MRD results [31]. Thus, it is crucial to look for new markers with stable expression during treatment that would strengthen the LAIP and facilitate reliable MRD detection in BCP-ALL. A review of the literature reveals many potential candidate markers. Coustan-Smith et al. performed a genome-wide gene expression study to evaluate several markers for detection of MRD in BCP-ALL, such as CD73, CD304, CD123, CD200, CD72, CD86, CD130. Out of these markers, CD73 showed significantly different expression pattern from normal hematogones, being at the same time one of the most frequently (around 55% of patients) overexpressed markers of blast cells. Some of these antigens (i.e. CD49f, CD69, CD132, CD83, CD79b, CD102, CD44) show similar expression on the hematogones, which excludes them as a potential markers for MRD detection [17]. Usefulness of CD73 was also reported by Tembhare et al., who compared expression of CD73 with CD86, CD72, CD44, CD200 and CD24 [14] and found overexpression of this marker in above 75% of patients. Also, Sędek et al. confirmed a high overexpression rate of CD73 on blast cells (≥ 2 nMFI value in 66% of cases, ≥ 7 in 26%), and stability during the early stage of treatment, as compared to CD304 and CD86 [15]. A different approach was described by Bras et al., who evaluated only one potential marker – CD123 – but on a wide group of acute leukemias, proving its variable utility depending on the disease type and age group [23]. Higher expression of CD73 on blasts as compared to normal hematogones was also demonstrated by Wang et al. [21]. Furthermore, in a study of Jain et al., CD73 overexpression on blast cells was observed in 90.41% of patients [32]. CD73 was also adopted by the EuroFlow Consortium and is included in the BCP-ALL MRD panel, but it is combined with CD304 in the PE position [10].
All these reports on differential expression of CD73 between normal and leukemic cells prompted us to further evaluate the stability of this marker during the course of therapy and to further explore its usefulness in MRD detection in BCP-ALL. In the current study, the stability of CD73 expression on leukemic blasts during the early stages of treatment was assessed, by comparing its expression level at diagnosis and days 15 and day 33 of induction treatment, with the use of a normalized expression scale based on MFI (nMFI), as described by Sędek et al. [15].Unlike in the 8-color EuroFlow BCP-ALL MRD panel, we decided to separate CD73 and CD304 markers to evaluate the input of CD73 only, without the possible influence of CD304, which was reported to have lower stability [15].
In the current study, a significant mean CD73 expression level increase was observed at day 15 of treatment, as compared to the initial diagnosis (mean of 19 and 5 nMFI, respectively), which is consistent with previously published data. Sędek et al. reported that in 95% of patients, expression of CD73 on blast cells was stable or higher at day 15 than at day 0 [15]. According to Wang et al. CD73 expression stability was proven in 5/9 (55.6%) cases, whereas in our study 164/177 (93%) patients showed stable or increased expression of CD73, which is in line with other published results [15, 21]. Moreover, this study also confirms the stability of CD73 expression at day 33 in patients who were still MRD-positive at that time point (the same mean nMFI as compared to day 15).
It is worth noting that in 6 cases CD73 expression rose from low nMFI values of 0-1 to much higher levels. Wang et al. also pointed out that the lack of CD73 expression on the day of diagnosis should not exclude CD73 from follow-up analysis, because quite often this expression is acquired during treatment [21].
The CD73 expression increase observed in the majority of cases was not correlated with any of the identified genetic subtypes of BCP-ALL except for hyperdiploidy. In patients with this numerical aberration significantly higher mean increase of CD73 expression was observed as compared with non-hyperdiploid patients (p < 0.01). In contrast, previous studies by Sędek et al. and Tembhare et al. did not identify any significant correlations between BCP-ALL genetic subtypes and the presence of CD73 overexpression on blast cells at diagnosis; however, in both cases the study group was smaller [14, 15]. Additionally, Wang et al. reported that patients with the BCR-ABL fusion gene presented higher expression of CD73 on blast cells, although this correlations was not statistically confirmed [21].
Comparison of CD73 to the other well-established markers confirms its significant utility as an additional antigen in blast cells’ separation from mature B cells. Since normal immature B-cell precursor cells are usually absent at days 15 and 33 of induction treatment, we could not use PCA for evaluation of separation of blasts from normal BCPs except for one case. Lower expression levels of CD73 on normal hematogones as compared to mature B-cells were however previously reported by Wang et al. and Sędek et al., which is a useful feature to distinguish leukemic cells from their normal counterparts [15, 21]. It is also important to point out that simultaneous use of several additional markers in a 10- or 12-color setting improves the sensitivity of MRD detection by FC, even if expression of some individual markers might not be present in a substantial proportion of BCP-ALL patients.
CD73 is a molecule of interest to many researchers. Expression of this molecule has been observed on many cancer cells of various origins [25, 26]. Its overexpression may be related to the increased capacity of the tumor to expand. In our research, we only checked the usefulness of using CD73 for MRD determination; however, according to Wieten et al., there is no significant correlation between CD73 overexpression and prognosis of ALL patients [33].
Conclusions
CD73 is a promising marker for MRD detection in BCP-ALL. It was proved that in the great majority of cases its expression is not only stable but increases during the therapy. This phenomenon is not frequently observed among other routinely assessed LAIP markers, which makes CD73 a very useful marker to be used for MRD monitoring in BCP-ALL patients.
The study was supported by a grant from the National Center for Research and Development, Strategmed 3/304586/5/NCBR/2017, “PersonALL”.