We report a case of the 67-year-old female patient with ulcerative colitis (UC), who was admitted to the Department of Digestive Tract Diseases in Norbert Barlicki Memorial University Hospital, Lodz for microbiota transplantation due to Clostridioides difficile infection (CDI) not responding to vancomycin treatment. Since 28 December 2020 she had been reporting abdominal pain, nausea, vomiting, and diarrhoea with mucus and blood (17–18 times/day). Additionally, the patient lost 5 kg during 3 weeks. Laboratory parameters showed elevated markers of inflammation: C-reactive protein (CRP) 108.00 mg/l (0–5), OB 52 mm/h (0–30), and iron deficiency: Fe 15.3 µg/dl (60–145). On 12 January 2021 the patient was admitted to the Regional Hospital in Konin for colonoscopy due to persisting symptoms. Until that time the patient had not been diagnosed with any gastrointestinal disease. Before examination, a SARS-CoV-2 RT-PCR test was performed, and the result was positive. Therefore, the colonoscopy was delayed, and on 14 January the patient was admitted to the COVID-19-appointed hospital ward in Słupca. During hospitalization the patient was treated with mesalazine, hydrocortisone, mebeverine, and pantoprazole. On 21 January she was transferred to another COVID-19-appointed hospital in Poznan for further diagnostics, where she stayed until 9 February. The patient was treated for COVID-19 with the following: ceftriaxone, enoxaparin, and dexamethasone sodium phosphate. A colonoscopy showed inflammatory lesions in the rectal colon segment and pseudopolyps in the rectum, sigmoid colon, and descending colon. In addition, there were ulcerations of mucosa in the rectum, sigmoid colon, and descending colon. A histopathological examination confirmed ulcerative colitis. There was also mild epithelial dysplasia. In the section from the sigmoid colon and rectum, active foci with inflammatory granulation tissue were confirmed. The material did not include the glandular epithelium. The patient was treated with prednisone and mesalazine.
On 12 February 2021 (to 25 February), she was re-hospitalized in the Department of Gastroenterology at the Poznan University of Medical Science in Poznan due to persistent symptoms. In colonoscopy the mucosa of the entire colon was swollen, brittle, and bleeding in the contact with ulceration. The rectal mucosa was red and swollen with yellowish white plaques. The whole picture indicated CDI, and so metronidazole treatment was introduced.
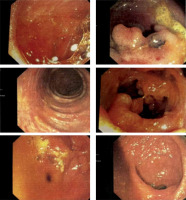
On 16 March 2021 CDI was confirmed with the Toxin A/B test. On 25 March 2021 the patient was admitted to the Gastroenterology Department in Konin. She was treated with vancomycin for 14 days (2 g/day) for CDI and mesalazine and hydrocortisone for UC. Nevertheless, the diarrhoea persisted. After consultation, the patient was discharged on 9 April and redirected to the Department of Digestive Tract Diseases in Norbert Barlicki Memorial University Hospital in Lodz. On 12 April a colonoscopy was performed, which confirmed the previous diagnosis of CDI and UC. There was a polypoid lesion in the orifice of vermiform appendix. The mucosa was swollen, hyperaemic with blurred vascular pattern along the entire length of the intestine, with numerous pseudopolyps, mainly in the descending colon and sigmoid colon (Figure 1).
Figure 1
Swollen, hyperaemic mucosa with blurred vascular pattern along the entire length of the intestine, with numerous pseudopolyps, mainly in the descending colon and sigmoid colon
Because of unsuccessful antibiotic therapy and recurrent CDI the patient was qualified for faecal microbiota transplantation (FMT). The procedure was performed and 100 ml of intestinal microbiota suspension (MBiotix HBI) was administered to the caecum: 50 ml proximal to the splenic flexure and 50 ml proximal to the hepatic flexure. No procedure complications were observed. Diarrhoea was relieved the day after the procedure. The patient is currently under follow-up with no C. difficile recurrence so far.
CDI is a serious clinical and economic burden. Patients with coexisting inflammatory bowel diseases (IBD) like Crohn’s disease (CD) and UC have an increased risk of numerous adverse events and longer hospitalization. Treating patients with CDI accompanying IBD represents a challenge because they are less likely to respond to conventional CDI medical therapy. In such cases, after antibiotic therapy (vancomycin or metronidazole), the relapse rate ranges from 22% to 34% [1]. The risk of CDI increases in patients with IBD chronically treated with steroids; these patients have an increased risk of colectomy, intestinal perforation, shock, respiratory failure, and death.
The exact aetiology of UC is unclear, and it is believed to be multifactorial with an interaction of genetic susceptibility, environmental factors, gut microflora, and an altered immune response. It has been suggested that an imbalance in the gut microflora significantly affects the progression of UC. Additionally, a meta-analysis showed that FMT is effective in mild to moderate UC in the short term [2].
Faecal microbiota transplantation is recommended for CDI treatment in patients with multiple recurrences, in whom targeted antibiotic therapy proved ineffective. FMT comprises the administration of a faecal solution from a donor into the intestinal tract of a recipient. FMT eradicates CDI and replaces missing components (live bacteria, bacterial components, and antimicrobial compounds of bacterial origin, e.g. bacteriocins or bacteriophages) of the microbiota [3] to increase bacterial diversity, similar to that of healthy donors. FMT as a non-standard therapy may be an effective treatment of CDI in patients with IBD. It normalizes intestinal dysbiosis, reduces gut inflammation in UC, and confers resistance to colonization with C. difficile.
Studies have shown that patients with UC who receive FMT may experience endoscopic, histological, and clinical remission. FMT in the treatment of IBD may be a promising but controversial treatment choice [4]. It can be particularly effective in UC patients after multiple administration via the lower gastrointestinal tract [5]. Numerous retrospective analyses of UC patients receiving multiple FMTs have shown few adverse events. The increased use of antibiotic therapy in the course of COVID-19 infections has a great impact on the weakening and depletion of the intestinal microflora, and thus increases the susceptibility to CDI infection. In addition, patients have limited access to medical care and delayed treatment for IBD.
A retrospective study conducted in 2020 found a 4-fold increase in the incidence of CDI in hospitalized patients during the COVID-19 pandemic (10.9% vs. 2.6% in the pre-pandemic period). The most significant risk factor was the use of antibiotics (other than azithromycin, e.g. ceftriaxone) due to complications of COVID-19. Other drugs, such as chloroquine or lopinavir/ritonavir, were not connected to the development of CDI [6].
A 2014 meta-analysis on a group of 122 patients (79 with UC, 39 with CD, 4 with unclassified IBD) showed an overall estimation of the achievement of clinical remission after FMT of 45% (54/119). Remission concerned 22% of patients with UC and 60.5% of patients with a CD [7]. Another study from 2015 showed that 24% (9/38) of patients with FMT-treated UC compared to 5% (2/37) of placebo patients achieved complete remission, defined as a combined Mayo score of 3 points and an endoscopic subscore of 0 points [8]. In 2019, the results of a multicentre study on a group of 73 patients with mild or moderate UC were published, demonstrating the efficacy and safety of repeated FMTs in maintaining endoscopic and histological UC clinical remission [9]. Finally, a study from 2020 on FMT usage in IBD showed that after FMT transplantation 62% (22/34) of patients with UC and 73% (11/15) with CD experienced clinical improvement in IBD. Additionally, among patients with UC and CDI relapses after FMT transplantation were seen in only 4% (1/34), and in CD patients there were no relapses [10]. Research results over the years show that the effectiveness of FMT in IBD is increasing.
Nevertheless, before the widespread use of FMT in IBD patients with CDI, multicentre, controlled, and randomized trials are necessary. The use of FMT to treat IBD is still an unconventional solution. A lot of research is being conducted that confirms the effectiveness of its use and shows a new possibility of treating IBD, especially when standard treatment methods do not bring improvement. We can assume that when the research will come to a controlled level, FMT will be used in the treatment of CDI in IBD, and probably even for IBD alone.










