INTRODUCTION
Central post-stroke pain (CPSP) is a form of chronic neuropathic pain, commonly seen as a complication arising in patients after stroke. The presentations of CPSP varies, most of them present with some abnormal pain sensation such as hyperalgesia, hyperesthesia, allodynia, dysesthesia, and more [1]. These sensory abnormalities can be continuously or intermittently felt by the patient. The onset of CPSP begins weeks to years following a stroke incident. One study reported an overall rate of CPSP in patients with stroke of any location at 11% [2]. Most of CPSP cases are diagnosed by exclusion, meaning physicians and patients both have to run off the list of possible causes of neuropathy before arriving at the diagnosis of CPSP [3].
The underlying mechanism of CPSP itself has not been clearly defined or sorted out. Previous studies offered different possible mechanisms behind this phenomenon. In general, previous studies found an association with the presence of abnormal pain pathways in the central nervous system (CNS). Various ideas had been suggested revolving around abnormalities in the central pain pathways, the spotlight being directed at the functional level of the CNS. CPSP might or might not present with detectable brain changes, but detecting the presence of abnormal or dysfunctional pathways might be the key to a strong and firm diagnosis [3, 4].
Recent advancements in neuroimaging technologies, particularly functional MRI (fMRI) and diffusion tensor imaging (DTI), have provided novel insights into the pathophysiology of CPSP. As a non-invasive radiation free tool, fMRI has the capacity to visualize patient’s brain metabolism and oxygen consumption in association to brain activity. As previous studies have stated that CPSP causes changes in the pain pathways in the brain, at the moment fMRI scans might be the best diagnostic tool to diagnose CPSP in patients with stroke. Comparing patients’ fMRI scans enables more precise identification of the neural networks involved in CPSP [5, 6].
Managing neuropathic pain is notoriously challenging for both physicians and patients. One study mentioned up to 63.2% of post-stroke patients experience pain, resulting in reduced quality of life [7, 8]. Effective disease management begins with the confirmation of a diagnosis. This literature review explores the role of fMRI and DTI in diagnosing and managing CPSP, emphasizing their potential to improve clinical outcomes by providing a better understanding of the neural mechanisms underlying post-stroke pain. By integrating these advanced imaging techniques, healthcare professionals can develop more targeted and effective treatment strategies.
STROKE AND CPSP IN CLINICAL PRACTICE
Stroke is defined as the development of neurological deficit as a result of acute focal injury of the CNS that is caused by a vascular pathology. World Health Organization provided a definition of stroke as rapidly progressive clinical signs of focal or total brain dysfunction, lasting more than 24 hours or leading to death, due to vascular pathologies [10].
The 2019 Global Burden of Disease Study identified stroke as the second leading cause of death, after ischemic heart disease, and the third leading cause of death and disability combined worldwide. The incidence and prevalence of stroke were 12.2 million and 101 million, respectively, with disability-adjusted life-years (DALYs) due to stroke reaching 141 million [11]. The average stroke-related medical cost is highest in the United States at $59,000 per patient per year [12]. Advances in medicine have reduced acute ischemic stroke (AIS) mortality, leading to more survivors with complications. Over 50% of stroke survivors experience impairments, including physical disabilities, psychological issues, and social impairments. These vary, based on factors such as age, hypertension, diabetes, National Institutes of Health Stroke Scale (NIHSS) score, stroke type, and marital status. Post-stroke complications can persist indefinitely, including seizures, incontinence, cognitive impairment, spasticity, hypertonicity, pain, and psychological issues [12].
Motor impairment following stroke has been relatively well-addressed compared to sensory complaints, which are often underreported and undertreated. Nearly 40% of patients continue to experience post-stroke pain five years after their stroke, impacting their quality of life and independence in activities of daily living (ADL). Post-stroke pain is categorized as nociceptive (e.g., musculoskeletal pain, shoulder pain, spasticity-related pain) or neuropathic. CPSP is the most common form of neuropathic pain in these patients. Diagnosing CPSP is challenging and time-consuming, as it requires excluding other pain sources, which can compromise patients’ quality of life [13-15]. Physicians must be aware of the development of any post-stroke disability and recognize it early. Post-stroke adaptations and quality of life rely heavily on the programmes created to improve patients’ rehabilitation process, promoting functional independence and improving overall well-being [16].
CENTRAL POST-STROKE PAIN
CPSP, formerly known as Dejerine-Roussy syndrome, presents itself as one of the most prevalent complications following an ischemic stroke. In the aftermath of the first- ever stroke episode, patients often present with complaint of pain. Investigating the underlying cause of pain in post-stroke patients is a considerable challenge, since the pain can be nociceptive or neuropathic or a mix of both. CPSP takes a form of a chronic neuropathic pain. Most often patients complain of abnormal sensory sensations such as pain, burning, prickling sensation, aching, stinging, and pins-and-needles-like pain. The onset of CPSP varies from a month to years after the stroke episode.
Initially after CPSP had been discovered, it was thought to happen only in patients with history of thalamic stroke. One study reported a pool prevalence of CPSP in patients with stroke at any location was 11%, while at least 50% of patients developed CPSP if the stroke had occurred in the medulla oblongata of the brainstem and the thalamus [2]. Recent studies found that CPSP could develop in patients with stroke anywhere in the CNS affecting the pain pathway and pain processing regions in the CNS. It is now accepted that any lesion occurring along the ascending somatosensory pathways can cause CPSP. One study reported a case of a 68 year-old-woman developing CPSP 5 months following an ischemic stroke in the right cerebellar hemisphere corresponding to the right posterior inferior cerebellar artery (PICA) [19, 20, 25-27].
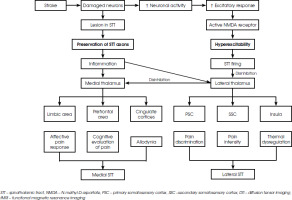
The perception and sensation of pain comes from two-way interaction, the ascending and descending pathways. The ascending path consists of two systems, the medial and lateral. The medial system is further constructed by different tracts, i.e., spinohypothalamic medial spinothalamic, spinoamygdalar, and spinoreticular tracts. Each of these will deliver information to the limbic area, prefrontal area, and cingulate cortices. The interpretation of this information in the aforementioned regions will yield a comprehensive empirical experience of pain, including the emotional aspect and also the responses to pain itself. On the other hand, the lateral system is the spinothalamic tract. It projects information to the lateral thalamus, which will later be conducted to the somatosensory cortices. The main function of the spinothalamic tract is the recognition, quantification, and mapping of nociceptive stimuli [24].
There are two recent theories explaining the development of CPSP. The first focuses on the presence of lesions along the ascending somatosensory pathways causing the generation of pain, whereas the second theory explores the imbalance between the excitatory and inhibitory inputs in the systems involved [28, 29]. It is very possible that in patients with CPSP both of these abnormalities occur simultaneously. When an injury happens to the spinothalamic tract, resulting in the preservation of spinothalamic tract instead of disruption, the damaged axons release inflammatory substances. These substances will act as a pain input to the adjacent healthy axons triggering hyperexcitation of the thalamus which increases the brain excitatory response and intercepts with its inhibitory regulation. This disinhibition alters the medial spinothalamic tract pathway, leading to burning sensation experienced by patients. Another theory about central sensitization, proposed the role of excitatory amino acids which send the N-methyl-D-aspartate (NMDA) receptors into overdrive. Increased activity of the NMDA receptors in the cortex or thalamic area will later produce spontaneous pain. One study found alterations in the fibre characteristics in the spinothalamic tract and superior thalamic radiation in post-stroke patients [28]. Lesions along the spinothalamic tract, seen in multiple sclerosis patients with plaques accumulating in the brainstem or thalamus, have been known to induce central neuropathic pain [29]. Figure I describes the development of CPSP in post-stroke patients and clinical manifestations corresponding to the affected spinothalamic tract [30].
One experimental study, successfully utilised repetitive transcranial magnetic stimulation (rTMS) to improve symptoms in patients with CPSP. This study highlighted specific metabolic alterations related to CPSP in the areas of stroke lesion [31]. Several treatment options for CPSP are currently offered, most of them being pharmacological therapies. Tricyclic antidepressants, serotonin-norepinephrine reuptake inhibitors, and gabapentinoids are almost always offered as the first-line therapy for CPSP [32]. In 2022, one meta-analysis reviewed the efficacy of antidepressants and anticonvulsants in the treatment of CPSP. The study concluded that gabapentin and pregabalin were the two most effective medications to improve pain in CPSP patients [33]. More sophisticated approaches are being also studied, such as sensory retraining, direct current stimulation, rTMS, deep brain stimulation, and even ganglion block using opioid [33-36]. These therapies are readily available in developed countries where healthcare is more accessible to everyone, and healthcare resources are not scarce.
CHALLENGES IN CPSP DIAGNOSIS
There are few studies aimed at providing the answer to when CPSP starts to develop in post-stroke patients. One study surfaced the idea that CPSP mostly develops within the first month, then the incidence rate drops as the time goes by [37]. Another systematic review found that CPSP develops within the first month after stroke in 31% of patients, while 41% of stroke patients develop CPSP between the first and twelfth month after the stroke. It was also mentioned that CPSP might develop even 12 months after the stroke in up to 5% of patients [2]. The wide range of onset does pose a big challenge for physicians to recognize CPSP in patients with stroke.
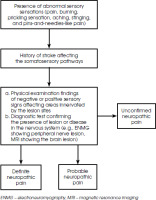
The International Association for the Study of Pain (IASP) defined neuropathic pain as the pain occurring as a direct consequence of a lesion or disease in the somatosensory system [20]. In 2011, The Neuropathic Pain Special Interest Group (NeuPSIG), proposed an algorithm to help diagnose neuropathic pain for both clinical and research purposes. One example of diagnostic workflow, modified from NeuPSIG guideline, can be seen below in Figure II. In CPSP, the affected area is commonly contralateral to the lesion in the central nervous system [30]. Neuroimaging is recommended to identify the lesion site; however, it is not used to confirm the diagnosis of CPSP [23].
Figure II
Diagnostic flow chart for neuropathic pain, modified from [52]. In clinical practice, a patient’s history and clinical examination may be sufficient to confirm the diagnosis without performing further testing
Currently available functional imaging modalities allow early monitoring of brain pathway alterations in stroke patients. A functional brain scan performed soon after stabilization of the acute stroke event can serve as a baseline for future comparisons [38]. During outpatient follow up; stroke patients should be offered a serial brain scan to be compared with the previous ones. Based on the previous study mentioned, screening for CPSP using fMRI should be carried out in the first 12 months after the first-ever stroke episode.
CPSP is frequently underdiagnosed due to its complex and multifaceted presentation, which often overlaps with other types of post-stroke pain or even unrelated chronic pain conditions. Many patients may not clearly articulate their symptoms, leading clinicians to misattribute their discomfort to musculoskeletal issues, postural problems, or unrelated neuropathies. Furthermore, CPSP can mimic other pain syndromes, such as central post-stroke spasticity or peripheral neuropathic pain, further complicating the diagnostic process. Without a standardized diagnostic framework in many clinical settings, CPSP remains largely unrecognized, leaving patients without adequate treatment or management strategies [4]. The difficulty in diagnosing CPSP is exacerbated by its reliance on subjective patient reports and the absence of definitive biomarkers or gold-standard diagnostic tools. While neuroimaging and functional studies can support the identification of central nervous system lesions, these modalities are not widely accessible in all clinical environments and are often underutilized. Additionally, CPSP’s delayed onset – sometimes appearing months or even years after the initial stroke – can be behind healthcare providers’ overlooking the condition as a potential cause of pain, particularly if the stroke history is not well-documented or considered [24].
Misdiagnosis is also common, as CPSP symptoms may be mistaken for other conditions, leading to inappropriate treatment plans. For example, pain originating from spasticity might be managed with muscle relaxants, which would be ineffective for CPSP. Similarly, antidepressants or anti-inflammatory medications might be prescribed under the assumption of a psychological or inflammatory origin of pain, respectively, leaving the central neuropathic pain largely unaddressed. This underscores the need for increased awareness among healthcare providers about the prevalence and distinct nature of CPSP, as well as the implementation of systematic screening and diagnostic protocols tailored for post-stroke patients [25].
FUNCTIONAL MAGNETIC RESONANCE IMAGING
fMRI is a relatively known neuroimaging tool consisting of imaging methods that employ MRI to visualize dynamic changes in cerebral tissue in response to neural metabolism [39, 40]. Functionally active areas in the brain are distinguished by a subtle signal increases, which enables high spatial and temporal resolution mapping of brain function [35]. Previously, the electric and magnetic signals resulting from neural activity have been studied using electroencephalography and magnetoencephalography, but there are limitations to in localizing neuronal signals, since the electrodes have to be placed outside of the skull [41, 42]. Using techniques such as positron emission tomography and single photon computed tomography makes it possible to acquire images of brain function but they rely on the use of radioactive tracers, restricting their use due to the possible long term side effects [43]. fMRI is a completely non-invasive alternative for functional imaging of the brain available for diagnostic approach [39].
fMRI tracks alterations in brain tissue oxygenation that reflect changes in brain tissue metabolism as a result of a task-based elicited neural response or spontaneous fluctuations in neural activity occurring when no conscious thought occurs (the resting state) [44, 45]. In the resting state, fMRI studies show brain-wide networks that originate from brain regions with synchronized, seemingly spontaneous activity. fMRI allows for the assessment of brain activity in response to sensory, motor, or cognitive tasks or stimuli [46,47] In task-based fMRI, time series data is evaluated according to a model of brain function that is predicted to correspond to the cognitive activity being carried out. The hypothesis can be supported or rejected for each voxel using statistical inference. This process creates a map of the brain areas that respond to the task, which can then be checked against phenotypical or genotypical models or parametric adjustments to the task, such as task difficulty [48].
Resting-state fMRI (rs-fMRI) is an alternative fMRI technique that evaluates spatial functional correlations within brain networks without requiring a stimulation paradigm [49]. Rs-fMRI has been used more often during the past decade to investigate changes in the brain’s intrinsic functional architecture as potential physiological correlates of neurological and psychiatric diseases [50-52]. The underlying premise in the resting state (RS) scenario is that specific brain areas with temporally synchronized fluctuations are linked as nodes of networks, such as the Default Mode Network [53, 54]. New networks continue to be identified every day [55, 56]. Data collection for RS follows similar procedures to those used for task-based studies.
THE BRAIN METABOLISM
fMRI is based on MRI, which in turn employs nuclear magnetic resonance in conjunction with gradients in the magnetic field to produce images that can incorporate many different types of contrast, including susceptibility, flow, T1 weighting, and T2 weighting, etc. [57, 58]. It is crucial to discuss brain metabolism first in order to understand the particular contrast mechanism predominantly used in fMRI.
Adenosine triphosphate is required as energy for all neural signaling processes in the brain, including action potential formation and propagation, vesicle binding at the pre-synaptic junction, emission of neurotransmitters across the synaptic gap, their reception and regeneration of action potentials in the postsynaptic structures, scavenging of excess neurotransmitters, etc. This nucleotide is primarily synthesized by mitochondria through the glycolytic oxygenation of glucose, with carbon dioxide produced as byproduct [59, 60]. Increased neuronal firing and other signaling processes that arise from a cognitive effort, like finger tapping, up-regulate (i.e., activate) the affected brain region, which in turn causes an increase in the cerebral metabolic rate of oxygen (CMRO2) in that area. A vasomotor response in arterial sphincters upstream of the capillary bed induces vessel dilatation as local oxygen (O2) stores in tissues proximal to capillaries are momentarily exhausted by glycolysis and waste product accumulation. The increased blood flow functions to restore the local O2 level required to overcome the temporary deficit; however, for reasons not yet fully understood, more O2 is transported than is required to counteract the rise in CMRO2 [61]. Therefore, neural up-regulation initially causes an increase in oxygenated hemoglobin (HbO2) and a decrease in deoxygenated hemoglobin (Hb) in the intra- and extravascular spaces. A subsequent vasodilatory response occurs within a second or two and overturns the situation, causing an increase in HbO2 and a decrease in Hb compared to the resting condition [62, 63]. This series of events constitute the hemodynamic response to neuronal activity. Hence, two main effects of increased brain activity can be observed on MRI: changes in oxygenation concentration (blood oxygen level dependent, or BOLD contrast) and an increase in local cerebral blood flow (CBF).
UTILIZED METHODS IN FMRI
Increased neural activity leads to two primary mechanisms detectable by MRI. The first mechanism detects the change in CBF that can be seen using an injected contrast agent and perfusion weighted MRI, as demonstrated by Belliveau [64], or non-invasively utilizing arterial spin labeling (ASL) [65]. Instead of being used for routine brain function mapping, ASL has been used to obtain quantitative measurements of baseline CBF for studies modeling the neurobiological activation mechanisms or calibration of vasoreactivity. However, compared to the BOLD contrast method, ASL is less sensitive, requires longer acquisition time, and is more sensitive to motion [62, 66].
The second mechanism, known as the BOLD contrast, was initially demonstrated in rats and later in humans [67-69]. BOLD contrast method is used in almost all traditional fMRI research. BOLD contrast is a result of the magnetic field around red blood cells changing based on the hemoglobin’s oxygenation condition. HbO2 is diamagnetic and magnetically identical to brain tissue when fully oxygenated, while fully deoxygenated Hb has 4 unpaired electrons and is extremely paramagnetic [70]. Local gradients in the magnetic field are produced by this paramagnetism, and their strength is influenced by the Hb concentration. By intravoxel dephasing and diffusion, respectively, these endogenous gradients modify the true tissue being imaged (T2) and observed tissue being imaged (T2*) relaxation durations of the intra- and extravascular blood. Using a gradient refocused echo (GRE) MRI pulse sequence, the acquisition is made sensitive to T2* and T2 [71, 72]. While the diffusion-weighted imaging (DWI) of T2 relaxation becomes more significant at higher field strengths, and offers greater spatial specificity due to signals being generated preferentially in capillaries and tissue with spin echo acquisitions, the T2* image is dominant at 1.5 Tesla and 3 Tesla and is largest in venules [73, 74]. Due to the higher T2* image, BOLD fMRI largely utilizes GRE techniques, which is appropriate given that the majority of fMRI is currently performed at 3 Tesla or below [75].
The recorded time series when fMRI is conducted can be invariably interrupted by non-neural sources of variations, such as head motion, physiological cycles, and magnetic field inhomogeneity, because fMRI measures brain activity indirectly via hemodynamic response to changes in metabolic consumption of oxygen. If left unchecked, these undesirable fluctuations may obscure the intrinsic patterns of neural activity, reduce the detection power of subsequent statistical analysis, or, in the worst cases, change the results of experiments by introducing structured noise that taints the actual results that are related to neural activity. Before conducting further analysis, it has been suggested to perform a number of computational steps, commonly referred to as the preprocessing pipeline, to eliminate the confounding sources of variation from the fMRI time series and boost the functional signal to noise ratio. The most frequently used steps are detailed as: quality assurance, slice timing correction, head motion correction, distortion correction, temporal filtering, spatial smoothing, physiological noise correction, functional-structural co-registration, spatial normalization, statistical analysis [73, 75].
DIFFUSION TENSOR IMAGING
DTI is a relatively new imaging method, developed from the existing DWI in MRI, intended to visualize the direction of the water molecules in a three-dimensional space. The data collected with DTI can later be processed to trace brain pathways. The process itself is more commonly known as tractography. The direction of the pathways connecting the brain areas can be assessed in fiber tractography. However, tractography cannot produce any functional aspect from the brain tracts, i.e. differentiating the afferent and efferent neurons in the same pathway [74].
Interpreting tractography results requires physicians to understand the relevant parameters. Some important parameters, essential to recognize sensory pathways in the brain, were previously studied and recognized. Since DTI measures the movement of water molecules, it produces four important diffusion parameters: fractional anisotropy (FA), axial diffusivity (AD), radial diffusivity (RD), and mean diffusivity (MD). Information from these parameters can be utilized to assess white matter integrity, including cell density, myelin, and axons [75,76].
FA values range from 0 to 1.0, with 0 indicating completely isotropic diffusion and 1 anisotropic voxel (one direction is usually more dominant). Voxel areas containing normal neurons typically produce values closer to 1, while lower values indicate possible neuronal injury [77]. Diffusivity of water molecules in DTI is further represented by AD and RD. AD indicates the diffusion aptitude of water molecules parallel to the tract within the voxel of interest, while RD represents the aptitude of water diffusion perpendicular to the tract. Hence, assessment of the axons and myelin sheath can be carried out by looking at the AD and RD, respectively [78]. MD measures the direction of molecules diffusion, especially water molecules in the brain. Increased MD signifies higher water content which typically correlates with less tissue resistance, and may indicate neuronal injury within the voxel [79].
Although DTI is not widely used in the clinical settings, some smaller-scaled studies have shown its sensitivity in detecting possible pathological and neurodegenerative changes in the brain’s white matter. One example of DTI potential is a study in which DTI tractography was performed in patients with mild traumatic brain injury, reporting a significantly reduced diffusion anisotropy compared to the control group [74]. DTI tractography has the potential to detect the presence of new brain pathways and/or alterations to pre-existing pathways.
THE ROLE OF FMRI AND DTI TRACTOGRAPHY IN DIAGNOSING CPSP
Current hypotheses on the pathogenesis of CPSP suggest alterations in the pain pathways of the CNS and/or the hyperexcitability of the pain pathways. fMRI can capture metabolic changes in the brain, with the BOLD method in fMRI visualizing brain metabolism by measuring blood oxygen level. Hypometabolism has been observed in the brain cortex areas associated with the pain pathways of the brain in CPSP patients [31]. Another study found an inverse correlation between pain intensity and the level of metabolism in the cerebral cortices in CPSP patients. It was reported that decreased metabolism was observed in patients experiencing increased pain intensity [80]. One study reported altered metabolism in different brain areas in patients with CPSP. Patients in CPSP group showed a significant decrease in metabolism in the precentral gyrus, postcentral gyrus, and contralesional cuneus, while a significant increase in metabolism was observed in the medial dorsal nucleus of the contralesional thalamus [81]. Another study suggested a reduction of functional connectivity involving brain areas related to the severity of motor impairment caused by particularly ipsilesional and/or contralesional areas of primary motor cortex [82]. According to a study conducted by Hosomi et al. [83], changes in functional connectivity of several brain regions play a role in pathophysiology of CPSP.
Alterations of the spinothalamic tracts were reported in patients with CPSP, which were seen in DTI tractography function [26]. Key parameters are the FA and MD. Changes in FA and MD can play a significant role in the diagnosis of CPSP. Increase in FA and decrease in MD relative to the baseline data indicate an increase in the fiber intensity of the pain pathway, while decrease in FA and increase in MD, relative to the baseline, indicate a decrease in the fiber intensity of the pain pathway. Less dense fibers in the pain pathway indicates preservation of partially damaged axons post-stroke, which can be the “pain generator” as they release pain inducing substance [29]. DTI tractography has proven effective in visualizing the fiber bundle changes. DTI tractography was effective in visualizing fiber bundle changes in CPSP, including decreased tract volume and partially damaged spinothalamic tract. Decrease in tract volume in itself correlated with partial damage of the spinothalamocortical pathway [84]. A DTI tractography study, comparing the integrity of the spinothalamic tracts of CPSP patients to healthy controls, found significant narrowing of the spinothalamic tracts in CPSP patients [85]. One study on a 59-year-old male presenting with pain increasing in intensity 6-12 months post-stroke, which was later diagnosed as CPSP showed DTI tractography results indicating significant reduction in FA and increase in MD, signifying the presence of tract damage and thinning [86,87].
These imaging techniques support the development of personalized rehabilitation programs, incorporating interventions such as neurostimulation, pharmacotherapy, and physical therapy aimed at modulating the altered pain pathways.
CONCLUSIONS
Combining fMRI and DTI data enables physicians to assess hyperexcitation and disinhibition in the somatosensory pathway, which are key factors in CPSP. Since CPSP typically develops within 12 months after a stroke, baseline data should be collected early. Follow-up screenings should identify pain symptoms suggestive of neuropathic pain. fMRI and DTI are essential for diagnosing and managing CPSP by visualizing brain activity and white matter integrity, respectively. These techniques help detect abnormal pain pathways and neural disruptions, facilitating early diagnosis, a better understanding of the condition, and targeted treatment. However, their limited availability, reliance on skilled technicians, and insufficient fMRI studies in CPSP remain as challenges. Future research should focus on expanding fMRI and DTI data in CPSP patients.
LIMITATIONS
A significant limitation of this study is the reliance on non-systematic methods for searching, identifying, and appraising evidence. This approach may introduce biases, limit the comprehensiveness of the findings, and affect the overall reliability and reproducibility of the results. To address this limitation, further studies should be employed. Utilizing standardized protocols, such as those outlined in systematic reviews and meta-analyses, will enhance the rigor and transparency of the research process. By adopting a systematic approach, future research can ensure a more comprehensive and unbiased inclusion of relevant studies, thereby improving the reliability and validity of the findings.