Despite the use of ablative doses in segmental transarterial radioembolization (TARE), also known as radiation lobectomy (RL), extrahepatic complications are extremely rare or underreported. This case report describes a hepatocolic fistula complicating RL.
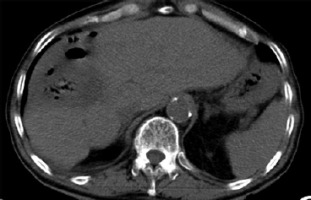
Ethics board approval for preparation of this case report was waived. A 74-year-old male patient with a history of rectal cancer and synchronous right lobe liver metastases underwent initial treatment with three lines of chemotherapy, a liver wedge resection, and a lower anterior resection. Three years later, a 9.7 cm right lobe colorectal liver metastasis (CLM) was identified, approximately 2.75 mm from the colon (Figure 1). The patient was referred to interventional radiology for consideration of locoregional therapy.
Figure 1
A – Baseline contrast-enhanced axial computed tomography (CT) with a 9.7 cm colorectal liver metastasis. B – Baseline contrast-enhanced coronal CT with the distance from the colon
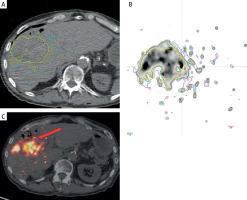
After multidisciplinary review, the decision to perform ablative RL with Y-90 resin microspheres (SIR-Spheres) was made. Pre-treatment work-up was performed and a dose of 500 Gy was planned for the tumor based on the partition model. Post-treatment dosimetry, performed using MIM Sureplan software, confirmed a mean absorbed dose of 463 Gy to the tumor, 215 Gy to the 5 mm margin, and 122 Gy to the 10 mm margin (Figure 2).
Figure 2
MIM software dosimetry analysis. Red arrow indicates absorbed radiation dose on post-Y90 PET/CT imaging, demonstrating its proximity to the colon
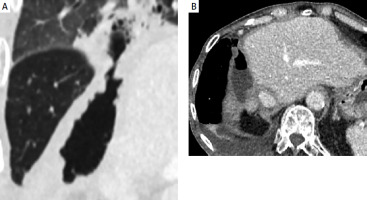
Three months after RL, the patient presented with fever and chills. Despite an infectious workup, imaging revealed a liver abscess secondary to a hepatocolic fistula (Figure 3). Intravenous antibiotics were administered, and a percutaneous drain was placed. Following a multidisciplinary team review, a right hepatectomy, fistulectomy, and colectomy were performed. Intraoperatively, a necrotic enteric wall with an abscess extending into the right liver lobe was identified. The patient was discharged on intravenous antibiotics 20 days after surgery with no signs of bacteremia.
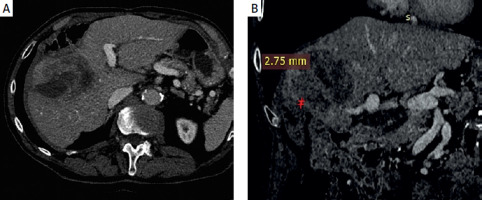
One month after surgery, the patient again presented with fever and chills. Imaging studies demonstrated a hepatopleural fistula (Figure 4). Due to the patient’s refusal of surgery, percutaneous drainage was performed. Repeated exchanges of the drainage were required over the following 3 years (Figure 5). The patient remained free of disease progression but ultimately died due to cardiovascular causes 3.4 years after RL.
Figure 4
A – Contrast-enhanced CT coronal plane showing hepatopleural fistula. B – Contrast-enhanced CT axial plane showing hepatopleural fistula with fluid and gas
Ablative TARE is an intra-arterial therapy that aims to administer a high, ablative radiation dose (> 190 Gy). Ablative TARE is associated with rare or underreported extrahepatic complications. To date, there is no study correlating the radiation absorbed dose with toxicity to nearby organs. Case reports have described bilioenteric [1], cholecystoduodenal [2], and gastrohepatic [3, 4] fistulae, after ablative TARE. These complications were not attributed to non-target embolization but rather to the irradiation of adjacent organs.
The close proximity of critical organs to target tumors presents a significant challenge in radiation-based therapies, including radiotherapy and TARE [4]. In the context of TARE, where the prolonged beta radiation emission from Y-90 can penetrate 1.1 cm, the protection of adjacent organs becomes paramount. The authors suggested that implementation of spacing techniques, inspired by practices in radiotherapy, could offer a promising approach to mitigate this risk and enhance the safety profile of ablative TARE [4].
Given the increasing use of ablative TARE and the availability of post-treatment dosimetry, a cautious approach is necessary when treating tumors near radiation-sensitive organs [5]. Patients with a history of surgery may be at increased risk due to limited organ mobility caused by adhesions.
With advancements in radiation protection techniques for adjacent organs, several strategies have been developed in prostate cancer radiotherapy to reduce rectal toxicity [6, 7]. Among these, the use of implantable perirectal spacers – such as absorbable hydrogels and saline-filled balloons – has been introduced to increase the distance between the prostate and the anterior rectal wall [6, 7]. This helps to lower the radiation dose absorbed by the rectum, thereby mitigating associated toxicity. These spacer techniques are generally safe, relatively easy to implement, and have demonstrated favorable outcomes in minimizing rectal exposure.
In the field of tumor ablation, a comparable approach known as hydro-dissection has traditionally been used to safeguard nearby organs when ablating tumors situated close to critical structures, such as lesions near the liver capsule [8]. This technique involves injecting fluid to physically separate and shield adjacent tissues from thermal damage during the ablation process.
However, the risk profile differs significantly when it comes to TARE. Unlike thermal ablation, which delivers short bursts of heat, TARE involves the delivery of yttrium-90, a radioactive isotope with a half-life of approximately 64 h. As a result, the need for sustained protection of adjacent organs is more pronounced. Given that Y-90 beta particles can penetrate tissue to a depth of up to 1.1 cm, careful attention must be paid to preserving nearby organs from prolonged radiation exposure. In clinical scenarios where high-dose TARE is anticipated and the irradiated liver tissue is in close proximity to other organs, incorporating spacer techniques may offer a valuable method to reduce the risk of radiation-induced injury.
In conclusion, this case report illustrates a hepatocolic fistula as a complication of ablative TARE. Given the potential for non-target organ irradiation, further studies may be necessary to identify high-risk patient populations and develop strategies to mitigate these risks.