Herpes esophagitis (HE) is typically caused by herpes simplex virus type 1 (HSV-1), although less frequently, it is caused by HSV type 2 (HSV-2). Most cases of HE occur in immunocompromised hosts, but occasionally immunocompetent patients develop this condition. Thus, HSV-2 esophageal infection in immunocompetent individuals is comparatively rare [1]. Canalejo Castrillero et al. performed a review of published HE cases (English and Spanish language) in immunocompetent patients from 1950 to 2009. The HSV type was identified in only 27 out 56 cases. However, among the identified cases, 96% (26) were due to HSV-1 and only 4% (1) was due to HSV-2 [1]. The overall reported incidence of HE is 1.8% [2]. Common risk factors for HE include a history of HIV, AIDS, malignancy, solid organs transplant, use of steroids, immunosuppressant medications, chemotherapy, and radiation therapy [1, 3–7]. An estimated 10–15% of bone marrow transplant patients and up to 20% of AIDS patients may develop HE [8]. The mode of esophageal infection in immunocompromised individuals is either viral reactivation or new infection, whereas in immunocompetent individuals, new infection is the primary mode [1].
We present a case of HE that is unique because it is due to HSV-2 in an immunocompetent patient who was found to have esophageal lesions, atypical for HSV-2 esophagitis on endoscopic visualization.
A 31-year-old woman with a history of intravenous drug use, anemia, and recent hip fracture repair after a motor vehicle accident presented with a right foot infection. She was admitted and initially treated with antibiotics. The hospital course was complicated by an episode of hematemesis and a decline in hemoglobin (HGB) from 7 mg/dl on admission to a nadir of 6.1 mg/dl (baseline HGB 10 mg/dl). She only noted some throat discomfort. She had no abdominal pain or tenderness on examination. A fecal occult blood test was positive. For the evaluation of a possible upper gastrointestinal bleed, she underwent esophagogastroduodenoscopy (EGD) which demonstrated an erythematous posterior oropharynx. Upper endoscopy also revealed multiple nonbleeding patches of linear ulcers involving the longitudinal folds of the proxima and distal esophagus (Figure 1). No source of active bleeding from the esophagus, stomach or duodenum was identified. Linear esophageal ulcers were thought to be due to cytomegalovirus (CMV) esophagitis on endoscopic visualization. Multiple biopsies of the esophageal ulcers were taken for diagnostic confirmation and she was empirically started on ganciclovir. Biopsy results revealed squamous epithelium with a viral cytopathic effect consistent with herpes simplex virus (Figure 2). Immunohistochemical staining was positive for HSV-2 and negative for Helicobacter pylori, CMV and HSV-1 (Figure 3). Based on the pathology results, ganciclovir was switched to valacyclovir for 2 weeks. A blood test for HIV was negative.
Figure 1
Upper endoscopy showing multiple injuries on the esophageal wall that were white linear ulcerative lesions surrounded by a non-erythematous base (white arrows); these lesions were prominent in the distal third of the esophagus, indicative of an infectious process
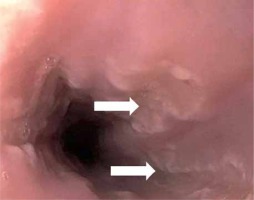
Figure 2
Histopathologic examination of the esophagus showing acute inflammation (black arrow) and ulceration with the presence of multinucleated cells (white arrow) consistent with herpetic infection (hematoxylin-eosin)
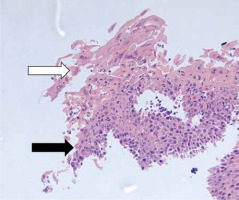
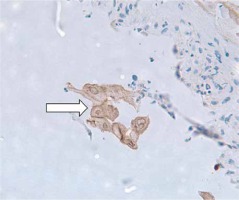
Figure 3
Immunohistostaining highlighting the presence of an HSV-2 viral particle (white arrow) within the esophageal epithelium revealing the infectious etiology surrounded by the acute inflammatory cells
The vast majority of HE patients are immunocompromised men with HSV-1 infections. In fact, HE is three times more common in men as compared to women [4–6]. The reason for this uneven distribution is unknown. Only a handful of HE cases due to HSV-2 have been published (Table I). Our patient reported throat discomfort which may have been part of a prodrome. Many patients do not experience prodromal symptoms such as fever, malaise, pharyngitis or respiratory symptoms [1, 3–6]. She was incidentally found to have esophagitis during an investigation for possible upper gastrointestinal bleeding. Typical presenting symptoms associated with HE include acute-onset odynophagia, dysphagia, nausea, vomiting, retrosternal chest pain and epigastric pain [1, 3–9]. Odynophagia is the most common presenting symptom in both immunocompetent and immunocompromised patients, accounting for slightly more than 75% of the cases [4].
Table I
Currently reported cases of Herpes simplex virus type 2 esophagitis in immunocompetent hosts
| Authors | Study year | Age [years] | Sex | Presenting symptoms | Predisposing risk factors | Endoscopic findings | Treatment |
|---|---|---|---|---|---|---|---|
| Naqvi et al. (current case) | 2020 | 31 | Female | Asymptomatic | None | Linear ulcerative lesions | Oral valacyclovir |
| Kurosawa et al. [14] | 2019 | 46 | Male | Sore throat Fever | Hemophagocytic lymphohistiocytosis | Multiple lesions | IV acyclovir and transitioned to oral valacyclovir for total of 21 days |
| Gani et al. [12] | 2019 | 29 | Female | Odynophagia Fever | Solid organ transplantation | White exudative lesions | IV acyclovir for total of 21 days |
| Kadayakkara et al. [3] | 2016 | 28 | Male | Dysphagia Odynophagia Epigastric pain | N/A | Small ulcers with exudate | IV acyclovir and transitioned to oral valacyclovir for total of 14 days |
| Hodnette et al. [13] | 2014 | 45 | Male | Dysphagia Odynophagia | AIDS | Black esophagus | IV acyclovir and transitioned to oral valacyclovir |
| Wishingrad [15] | 1999 | 32 | Male | Odynophagia Fever Retrosternal pain | N/A | Multiple superficial ulcers | N/A |
| Wandl-Huinberger et al. [16] | 1998 | 36 | Male | Dysphagia | Homosexuality | Multiple small ulcers | N/A |
Globally, HSV-1 is a common cause of orolabial infections, whereas HSV-2 is more frequently associated with genital infections [10]. In patients with HE due to HSV-1, herpes labialis will be present in 20% of cases prior to the onset of odynophagia [3, 5, 6]. Irrespective of the type, HSV from the oral cavity or pharynx is thought to be transmitted to the esophagus, where it invades the esophageal mucosa and results in ulcerative lesions. HSV has a predilection for traumatized esophageal mucosa that may result from gastroesophageal reflux disease or even from endoscopic interventions [1]. The distal esophagus is the predominant location of HE (68.3%); however, any portion of the esophagus and even the stomach can be infected [1, 3–6, 9]. The characteristic findings on endoscopic visualization are multiple, discrete small vesicles [1, 3–7, 9]. The lesions can be divided into two stages based on lesion characteristics [3–5]. In the early stages, small vesicular lesions are usually visualized on EGD; these may eventually slough off and become circumscribed ulcers. In the late stages, necrosis of esophageal mucosa may be seen. Punched out lesions are pathognomonic of HE and likely represent a transitional stage [9]. To confirm the diagnosis, brush border cytology or biopsies should be obtained from the ulcer edges [1, 5]. Cowdry inclusion bodies (eosinophilic intranuclear inclusion bodies) and giant multinucleated cells are diagnostic of HSV in only 67% of cases [1, 4, 5, 7]. Although the traditional gold standard test is virus isolation from cell culture, polymerase chain reaction (PCR) of the HSV DNA has the highest sensitivity (92–100%) and specificity (100%) [5, 11]. In our case, confluent linear ulcers were noted on EGD from the oropharynx to the distal esophagus. Tissue samples revealed a viral cytopathic effect. Given the initial endoscopic appearance, there was a concern for CMV esophagitis. However, immunohistochemistry confirmed an HSV-2 infection and ruled out HSV-1 and CMV.
Only a few of the published HSV-2-associated esophagitis cases presented with dysphagia or odynophagia, unlike most HSV-1 cases (Table I) [3, 12–16]. The majority of reported cases presented in the third to fourth decade of life with male predominance. Organ transplantation is the most common predisposing risk factor for HE, followed by AIDS and hematological malignancies (Table I). Upon endoscopic evaluation, patients were found to have multiple superficial lesions. Complete resolution of lesions with a 2–3-week course of intravenous and oral antiviral treatment was reported.
HE in immunocompetent individuals is a self-limiting disease [1, 4]. In a meta-analysis of 56 immunocompetent patients with HE, 30 patients received no antiviral therapy. Resolution within 2 weeks is reported in the majority of studies [3, 4, 6]. Since there are no randomized controlled trials (RCTs) on the management of HE in immunocompetent patients, it is unclear whether treatment with antiviral medication hastens recovery. However, treatment to avoid severe complications such as upper gastrointestinal hemorrhage or esophageal perforation in high risk patients seems reasonable [4]. In our case, the patient was treated with a 2-week course of valacyclovir, resulting in complete resolution of her lesions.
In conclusion, Herpes esophagitis is usually due to HSV-1 and typically occurs in immunocompromised individuals. However, esophagitis due to HSV-2 in immunocompetent individuals does occur and patients may present without classic odynophagia or retrosternal chest pain. A sore throat in the proper clinical context could be a clue. Thus, a high index of clinical suspicion is required to diagnose HE in immunocompetent patients. Upper endoscopy with biopsy of esophageal mucosal lesions is an essential modality for the diagnosis. Immunohistochemistry and PCR from tissue samples are diagnostic tests with a high sensitivity and specificity. Although RCTs are lacking, it seems reasonable to treat immunocompetent HE patients with a course of antiviral medication to reduce the incidence of complications.









