INTRODUCTION
Asthma treatment, as outlined by most standards, initially involves the use of anti-inflammatory medications (inhaled corticosteroids – ICS) combined with bronchodilators (short-acting β2 agonists – SABA, or long-acting β2 agonists – LABA), depending on the level of asthma control and severity of symptoms. According to the Global Initiative for Asthma (GINA) guidelines, asthma management follows two treatment pathways: the preferred and alternative approaches. The preferred option remains the use of a single inhaler containing ICS and formoterol as the primary treatment. This approach is favoured due to its simplicity, reduced risk of exacerbations, and the ability to adjust the anti-inflammatory dose based on current symptom severity. Patients with Step 1 or 2 asthma should use medication only as needed. From Step 3 onward, Maintenance and Reliever Therapy (MART) is preferred. MART involves the use of additional ICS/formoterol (ICS/FF) doses as needed for symptom relief. For Step 4 asthma, treatment (depending on the pathway – preferred or alternative) includes medium- or high-dose ICS + LABA. If asthma remains uncontrolled, escalation to triple therapy (ICS/LABA/LAMA)(long-acting muscarinic antagonists) – preferably in a single inhaler (single inhaler triple therapy – SITT) is recommended. GINA 2024 provides room for clinical decision-making regarding whether to increase the ICS dose in a combination inhaler containing ICS/LABA for patients on medium-dose inhaled steroids or to add LAMA to the current treatment regimen. These considerations are the main focus of this article.
BENEFITS OF LAMA USE IN ASTHMA
The benefits of triple therapy (ICS/LABA/LAMA) for asthma patients are summarised in Table 1 [1–5]. The primary effect is a reduction in the rate of severe exacerbations without any noticeable change in the treatment regimen from the patient’s perspective. The patient has been taking inhaled medications and continues to do so (with their inhaled medication escalated to triple therapy from a previous dual therapy regimen). They continue using the same inhaler they are accustomed to, and no additional medications are used. Thus, their medication adherence is unaltered, but the frequency of severe exacerbations is significantly reduced. The majority of asthma patients do not complain of dyspnoea or wheezing but rather of excessive mucus production, which forces them to cough up sputum. This leads to considerable discomfort for patients. Triple therapy significantly reduces bronchial mucus production. This is one of the fundamental mechanisms of anticholinergic treatment. The mechanisms of action of LAMA in bronchial asthma have been described in Table 2 [1, 3, 6, 7]. Undoubtedly, reducing mucus production and significantly lowering the number of asthma exacerbations improve the quality of life. In many patients, triple therapy makes it possible to avoid or significantly delay the initiation of biological treatment. Without a doubt, these are the most important benefits that asthma patients can experience in clinical practice. Due to the mechanisms of action described above, there is a high likelihood that these medications will soon be used in the treatment of patients with less severe asthma symptoms as well.
Table 1
Table 2
ASTHMA PHENOTYPE AND TREATMENT OF UNCONTROLLED ASTHMA
There is a lack of studies attempting to define the phenotype of patients who respond better to LAMA or to an increased dose of ICS. Since most studies are typical clinical trials designed to assess the clinical efficacy of drugs rather than focusing on identifying phenotypes, they are difficult to interpret conclusively.
The role of extrafine-particle ICS/LABA/LAMA combination therapy in achieving asthma control was demonstrated in clinical trials published 4 to 5 years ago [8–11]. Since then, numerous additional studies and meta-analyses have provided further evidence supporting its benefits [12].
Adding LAMA to treatment with medium or high doses of ICS/LABA has been shown to improve lung ventilation parameters [8]. This is particularly evident in patients with fixed airflow obstruction [8]. Similarly, the introduction of SITT in patients previously treated with medium- or high-dose ICS plus LABA has been found to reduce the number of exacerbations. According to a meta-analysis, such therapy decreases the frequency of severe exacerbations by 17% [13]. A significant improvement in asthma control was also observed [13]. Phenotypic analysis revealed that patients with a history of exacerbations in the preceding year (exacerbation-prone phenotype) derived the greatest clinical benefit in terms of reduced exacerbation frequency. These findings suggest a broadly universal therapeutic effect of LAMA add-on therapy across the studied population.
Identifying patient subgroups most likely to benefit from triple therapy has become a focus of multiple clinical studies. Post-hoc analysis of the TRIMARAN and TRIGGER trials demonstrated that in patients with persistent airflow limitation (PAL; post-SABA FEV1/FVC ≤ 0.7), triple therapy significantly reduced the rate of severe exacerbations compared to ICS/LABA dual therapy (23% in the overall population, 33.5% in the PAL subgroup). Treatment also prolonged the time to the first severe exacerbation [9–11].
It is important to note that current clinical practice in some countries involves initiating triple therapy immediately following the first moderate asthma exacerbation in patients receiving dual therapy [14].
TRIPLE THERAPY DEMONSTRATES GOOD TOLERABILITY
An analysis of adverse effects indicates that triple therapy is better tolerated than treatment with a high-dose ICS/LABA combination [2, 6, 15, 16]. The main, though uncommon, side effect of SITT is dry mouth, which is directly linked to the mechanism of action of LAMA [2]. It is important to note that higher ICS doses increase the risk of adverse effects, mainly localised to the throat and larynx. GINA 2024 recommends short-term intensification (3–6 months) to high-dose ICS when current medium-dose ICS/LABA combination therapy fails to achieve asthma control and other therapeutic options (such as biologic therapy) are difficult to implement. Short-term treatment intensification may also be considered as a preventive strategy during periods of increased exacerbation risk, such as seasonal allergen exposure or peaks in viral respiratory infections. Temporarily increasing ICS doses during these critical periods may help reduce the frequency and severity of asthma exacerbations, particularly in patients with documented seasonal allergen hypersensitivity or frequent infections. This approach may improve disease control and reduce the need for oral corticosteroids during severe exacerbations.
SEVERE ASTHMA – TRIPLE THERAPY
Approximately 40% of patients with severe asthma receive combination therapy, either with or without biologic agents [3]. Some publications indicate that 35.9% of severe asthma patients are treated with regimens including LAMA medications, which suggests that a substantial patient population does not currently receive triple therapy [17]. Currently, there are no available data on the use of triple therapy for asthma management in Poland. Patients with severe asthma who undergo triple therapy are typically older, have a later onset of the disease, are smokers or former smokers, and exhibit poorer lung function [4, 18]. In clinical practice, the persistence of asthma exacerbations despite high-dose ICS/LABA treatment emerges as the most significant determinant for initiating triple therapy [18]. For severe asthma cases, implementing triple therapy before considering biologics may represent an effective, more cost-efficient, and often more convenient therapeutic alternative.
In Poland, patients with severe asthma are frequently prescribed long-term oral corticosteroids, often for many years. According to GINA 2024 guidelines, such treatment should be a last resort, and patients should undergo a thorough evaluation regarding diagnosis, comorbidities, and other therapeutic options.
WHAT DO PHYSICIANS THINK ABOUT TRIPLE THERAPY?
The decision to initiate therapy ultimately lies with the physician. Their perspectives on the application of specific therapies provide valuable analytical insights. Bagnasco et al. present an analysis of physicians’ opinions and reasons for using or discontinuing triple therapy. Among the surveyed physicians (allergists, pulmonologists, and paediatricians), 35.7% believed that triple therapy was appropriate for treating their patients, while 61.8% considered it an option only after failing to achieve asthma control with high-dose ICS/LABA. Only 18.5% of physicians disagreed with this view [7]. Physicians were most likely to prescribe triple therapy for patients with low ventilatory parameters (20.1%) and small airway dysfunction (16.4%). Interestingly, the presence of exacerbations as a factor necessitating triple therapy was not explored in this study [7].
EXPERT RECOMMENDATIONS
The 2024 expert recommendations from the Polish Society of Allergology (PTA) and the Polish Respiratory Society (PTChP) highlight the following role of triple therapy in asthma management:
it significantly reduces exacerbation rates in patients who remain inadequately controlled on medium- or high-dose ICS/LABA,
it improves quality of life and allows for achieving asthma control in patients with previously uncontrolled disease,
it effectively improves pulmonary ventilation parameters regardless of baseline characteristics such as gender, age, allergic status, disease duration, age at asthma diagnosis, and severity of bronchial obstruction,
it proves effective for patients with irreversible airflow limitation, including those with mild asthma, and remains beneficial even in cases of asthma associated with T2 inflammation,
it significantly improves adherence to treatment in the patient population,
it reduces healthcare costs and decreases the need for biologic therapy [19].
The latest recommendations from the European Academy of Allergy and Clinical Immunology (EAACI) experts emphasise similar benefits of triple therapy, highlighting a predominant trend toward reducing exacerbations and advantages for patients with T2 inflammation who do not achieve full control with ICS/LABA treatment [20].
CONCLUSIONS
The author of this work argues that the initiation of triple therapy is largely driven by the occurrence of exacerbations. Therefore, in patients receiving at least medium-dose ICS/LABA therapy, this specific clinical factor should prompt the initiation of ICS/LABA/LAMA while maintaining the current ICS dose. Given the non-linear dose-response relationship of ICS on lung function improvement (especially at high doses) and their associated adverse effect profile, adding LAMA to medium-dose ICS/LABA represents a preferable strategy over ICS dose escalation. Recent clinical studies appear to confirm the rationale for this therapeutic approach. Triple therapy provides benefits for patients with uncontrolled allergic asthma and irreversible airway obstruction [21–24].
A holistic approach to patient management remains essential. Inflammatory biomarker levels and other clinically relevant factors should be evaluated to guide optimal therapeutic decisions [25].
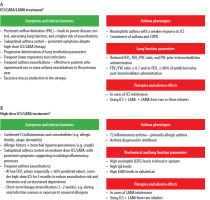
The PTA and PTChP Expert Panel recommends the following approach:
“For adult patients failing to achieve optimal asthma control on medium-to-high dose ICS/LABA therapy (irrespective of GINA-recommended administration strategy − Pathway 1 [MART] or Pathway 2), the preferred treatment intensification strategy should be LAMA addition, optimally administered as Single Inhaler Triple Therapy (SITT) (Figure 1). This indicates the preference for SITT over other options for asthma treatment escalation, including: increasing ICS dose, adding LTRA, adding SAMA, including oral steroids, initiating biological treatment.”