INTRODUCTION – CASE REPORT
A 46-year-old right-handed male with a history of hypertension, lipid metabolism disorders, and fatty liver with elevated transaminases was admitted to the hospital in September 2023 due to suspected stroke. He felt unwell at work, and experienced anxiety associated with chest pain, occipital headache, unclear speech, and weakness in the right limbs which were sudden in onset. He could not walk independently, prompting the call for an emergency medical team. On the day of admission, he reported a history of four ischemic strokes and transient ischemic attacks (TIA) (each time with right limb weakness). In recent days, he consumed more alcohol than usual due to stress, a few beers daily. On physical examination, he was conscious, maintained verbal-logical contact but displayed right-sided limb weakness: 4th degree on the Lovett scale, National Institutes of Health Stroke Scale (NIHSS) – 3. When distractors were applied, his limbs did not sag before the specified time. Rankin scale before the onset of illness equalled 0. Non-contrast head computed tomography (CT) showed no haemorrhage or evidence of early infraction with an Alberta Stroke Program Early CT Score (ASPECTS) of 10 points. For technical reasons, the diffusion-weighted magnetic resonance imaging (DWI-MRI) was not performed at admission.
The patient was initially considered for thrombolytic treatment but he declined and confirmed his refusal in writing. During further observation in the stroke clinic, some inconsistencies in the reported weakness was noticed – the patient, still presenting upper limb weakness, signed a refusal demonstrating efficient holding of a pen. The nursing staff reported an incident when a bee flew into the room, and the patient instinctively waved his right limb in a manner inconsistent with the presented weakness. He claimed that the weakness had subsided the following morning, which made it impossible to perform the functional weakness tests. Additionally, the neurological examination highlighted tremors in the upper limbs.
After completing the interview and review of the medical documentation, it was revealed that the patient had been hospitalised in different neurological clinics at least eight times over the last ten years, always with the diagnosis of ischemic stroke or TIA (October 2013, March 2015, May 2016, 2017, January 2019, June 2019, 2021). His available medical records showed that the thrombolytic therapy was administered three times. However, at least three discharge summaries from the hospital were missing. The hospitalisations were consistently due to right limb weakness, varying in severity, sometimes accompanied by speech disturbances, headaches, dizziness, and gastrointestinal symptoms. During the 2019 hospitalisation, a consulting psychiatrist suggested the possibility of dissociative disorders or simulation. During the penultimate hospitalisation, the diagnosis was of “suspected somatisation disorders with transient speech disorders and limb weakness in a patient with vascular brain damage”.
Extensive cardiac (including transoesophageal echo), hematologic, rheumatologic and genetic diagnostics have been performed – identifying heterozygous C/T polymorphic variant c.677C>T of the methylenetetrahydrofolate reductase (MTHFR) gene and heterozygous A/C variant c.1298A>C of the MTHFR gene (associated with decreased MTHFR enzyme activity and predisposition to mild hyperhomocysteinemia and cardiovascular diseases). No Leiden mutation in the F5 gene or c.20210G>A mutation in the F2 (prothrombin) gene was found, and biochemical tests ruled out Fabry disease.
In the latest head MRI (April 2022), minor hyperintense foci in the white matter of both hemispheres were observed, likely representing chronic vascular changes. Previous brain MRIs, while limb weakness was observed, had never showed acute ischemic lesions, similarly to the current hospitalization.
The subsequent examination revealed many recent stressful situations in the patient’s life. He reported that his physical complaints were escalating; perceived a connection between the timing of stressful incidents and hospitalisation in various neurological departments.
The Minnesota Multiphasic Personality Inventory-2 (MMPI-2) questionnaire was administered during the psychological assessment. The validity scales allow for the interpretation of the profile (valid profile): on the Variable Response Inconsistency Scale (VRIN), the T-score result was 55, and on the True Response Inconsistency Scale (TRIN), it was 74F. The T-score on the TRIN scale, although indicating a valid profile, also suggested a certain tendency to deny difficulties related to mental health. The remaining validity scales, in addition to confirming the valid profile, indicated that the patient might tend to minimize symptoms.
The interpretation of the clinical scales indicated dominant, strong anxiety, including health anxiety, a tendency to somatise symptoms, and a lack of full insight into the manifestation of difficulties on the psychological level (high scores obtained on the Hypochondriasis Scale: T-score 64, and Hysteria Scale – 68, elevated score on the Psychasthenia Scale: T-score 66, and the Paranoia Scale – 60). Furthermore, the patient might have tended to present elusive symptoms that can appear and disappear and respond with somatic symptoms to stressful situations. The patient also presented lowered mood that did not take the form of clinical depression (elevated score on the Depression Scale: T-score 69). Harmful use of alcohol could be regarded as a way to regulate emotions through escapism. In conclusions, the psychological examination revealed high probability of somatisation in response to stress, and a tendency to downplay psychological difficulties which could lead to underestimating emotional distress.
The patient was discharged with a diagnosis of functional neurological disorder with transient right limb weakness. All of his symptoms that led to hospitalisation resolved early. Follow-up care in the Mental Health Clinic was recommended, focusing on psychological or psychotherapeutic support. The Alcohol Use Disorder Identification Test (AUDIT) was recommended.
Purpose
When it comes to patients with new neurological deficits, sometimes the diagnostic decision can be extremely challenging. There is pressure on neurologists to treat suspected acute ischemic stroke (AIS) patients with recombinant tissue plasminogen activator (rtPA), as the failure to treat eligible patients is one of the most frequent stroke-related malpractice claims [1, 2]. Additionally, the time pressure is also linked to the length of the therapeutic window. These factors increase the risk of treating stroke mimics (SM), including functional stroke mimics (FSM) with intravenous tissue plasminogen activator (ivtPA) with an upward trend of 3.5% to 4.1% [3-5]. Some authors suggest that the incidence of thrombolysis treatment of SM can be as high as 17% [6]. It is important therefore to diagnose SM patients properly and provide them with appropriate treatment. The article aims to summarise the knowledge of FSM and thrombolytic treatment and propose a structured algorithm for FSM management.
METHODS
A comprehensive literature search was performed involving PubMed, SCOPUS and Google Scholar with Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines to identify the articles that discussed FSM. The keywords used were “functional stroke mimics”, “functional neurological disorders”, “thrombolytic treatment”, “ischaemic stroke”, and “functional weakness”. A sequential literature search was conducted, with a separate search for each section. The relevant articles have been cited. No limit has been placed on the publication time.
Views
Definition and diagnostic criteria of FSM
FSM is a functional neurological disorder (FND) presenting as a stroke. FND was previously known as psychogenic or conversion disorder [7]. Although Freud already described conversion disorders as an unacceptable psychological conflict leading to the conversion of psychological stress into physical symptoms, providing a practical definition for FND proved challenging [8]. The term “conversion disorder” in the “Diagnostic and Statistical Manual of Mental Disorders, fourth edition, text revision” (DSM-4-TR) refers to symptoms like weakness, epileptic-type attacks, abnormal movements or sensory disturbances that cannot be linked to physical nerve damage or intentional deception but are believed to be connected to psychological influences [9]. In practice, the psychological factors are often not identified [10]. Therefore, criteria related to the impact of psychological factors and the obligation to exclude malingering were abandoned in the latest DSM-5-TR. “Conversion disorder” was retained in DSM-5-TR. It was renamed “functional neurologic symptom disorder” (FNsD), a subset of FND. Diagnostic criteria for FND are as follows: (1) one or more symptoms of altered voluntary motor or sensory function; (2) clinical findings can provide evidence of incompatibility between the symptom and recognised neurological or medical conditions; (3) another medical or mental disorder does not better explain the symptom or deficit and the symptom or deficit results in clinically significant distress or impairment in social, occupational, or other vital areas of functioning or warrants medical evaluation [11]. These criteria stress the importance of positive clinical evidence to support the inconsistency of symptoms rather than relying solely on the absence of medical findings. Many guidelines for FND were established through collaboration among neurologists, psychiatrists, and other healthcare professionals [12-15]. In practice, we may also use the definition of FND proposed by Hallet et al. [10], of “clinical syndromes consisting of symptoms and signs of genuinely experienced alterations in motor, sensory or cognitive performance, which are distressing or impairing and manifest one or more patterns of deficits that are consistent predominantly with dysfunction of the nervous system and show variability on performance within the same task and different tasks”. Because this definition captures the essence of FND simply and practically, the authors suggest that it is worth using it in everyday practice.
Various indicators in the literature may suggest the diagnosis of FSM: (1) lack of compliance with typical neurological symptoms, (2) unusual symptom presentations, (3) variable nature of symptoms, (4) presence of psychological factors, (5) the existence of emotional or situational triggers, (6) background of previous medical issues that have been examined in the past without evident improvement, (7) a lengthy history of repetitive TIA/stroke or history of repeated hospitalisations [16].
All the above criteria and indicators for FND can also be applied when diagnosing FSM. The term FND emphasises that the disorder involves a disturbance of function rather than damage to structure. Additionally, FND is an emotionally and causally neutral term in opposition to ‘psychogenic’, ‘psychosomatic’ or ‘somatisation’, which refer exclusively to psychological causes. The term ‘functional’ could make accepting the diagnosis more accessible to the patient. It is essential, from a practical perspective, that FND symptoms often result in serious disability for the patient.
Epidemiology of FND, SM and FSM
FND is frequent in neurological settings and refers to 5-15% [17, 18] of new cases in neurology clinics. Other population studies indicate a prevalence of 50-100 cases per 100,000 [19-21] and an incidence of 12 cases per 100,000 per year [22]. Depending on the source, various data on the frequency of recognising SM can be found in the literature. According to the National Institutes of Health (NIH), it may be 9-31% of stroke patients [23]; depending on the setting and methodology, this percentage may reach up to 47% [24-29]. The frequency of SM depends on whether the patients are evaluated by the emergency personnel or stroke physicians, the figure being higher in the second case [1, 30, 31]. SMs are defined as medical mimics and FSM [32]. Considering this division in large case series, it has been noted that FSM were generally less common, accounting for 20-50% of SM [31, 33]. However, FSM may be found in as many as 6.4% [26, 34, 35] to 8.2% of all stroke presentations [36]. In a group of 802 Polish patients with the initial diagnosis of acute cerebrovascular event, SM were diagnosed in 32.2% following thorough neurological assessment. The authors described no FSM in their study population. The frequent medical SM were vertigo, headache, seizures, blood hypertension, electrolytes and metabolic disturbances, infections, and syncope [37].
In the case of acute focal neurological symptoms, SM are “false positive” diagnosis, which refers to a condition suggesting the presence of a stroke despite its absence. In contrast, stroke chameleons, are defined as a “false negative” diagnosis, where the clinical presentation suggests another disorder despite the occurrence of a stroke [33]. Failure to diagnose a stroke may occur when the onset of the stroke is atypical, for example, when symptoms progress gradually, when there is an unusual clinical presentation, when a single lesion does not explain the clinical findings, or when symptoms, such as paraesthesia, dizziness, or seizures are ‘positive’ [25, 38]. Stroke chameleons may also present with headaches and be incorrectly diagnosed as having a benign primary headache disorder like migraine [39].
The consequences of stroke chameleons are more severe than SM. They might be detrimental, as patients miss proper treatments during the acute phase of stroke and for secondary prevention, leading to generally poorer outcomes [33]. However, the missed diagnosis of strokes occurs less frequently compared to SM diagnosis. According to the literature review by Liberman et al. [25], the rates of stroke underdiagnosis (false-negative cases) range from 2% to 26%.
FND mechanisms
Although FND is frequently diagnosed, its fundamental mechanisms are poorly understood. In FND, attention dysregulation is considered a key feature [40]. Physiologically, a hierarchical flow of information occurs, meaning the integration of bottom-up sensory information with top-down predictions regarding the nature of expected sensory input [41]. In FND, the prediction of sensory information is aberrant, with misplaced attention focused on the body, driving abnormal perceptions or movements [40]. This mechanism explains symptom suppression through attentional diversion, serving as a diagnostic criterion and the basis for new physiotherapy techniques [42, 43]. Recent research has revealed that there are no psychological differences between FND patients and those with neurological movement disorders [44]. Additionally, many FND patients show no abnormalities in psychological assessment conducted with screening tests [44]. In cases of functional weakness and paralysis, the motor system, spanning from the motor cortex to the muscles, appears unaffected. The effective treatment of FND is unknown which results in poor long-term outcomes and prognosis with significant levels of physical impairment [45].
Clinical implications
Symptoms and neurological assessment of FSM
Acute onset of motor and sensory deficits and speech and language disorders could be symptoms of FSM. Functional weakness and sensory disturbances are among most common symptoms of FSM and comprise about 70% of all cases [46]. Functional motor or sensory deficits are typically lateralised [47]. A detailed subjective examination should precede the neurological assessment. The presence of multiple symptoms that fail to adhere to well-defined stroke syndromes and a history of several functional symptoms should be red flags for FSM. Symptoms cannot confirm the anatomical localisation (cortical vs. sub-cortical) and vascular distribution (anterior vs. posterior circulation) [32, 48].
The key finding in the clinical examination of a functional weakness is inconsistency, especially with repeated examinations. Symptoms are usually atypical and fluctuating [49, 50]. The diagnosis may be assisted by observing the patient presenting with functional weakness, who could quickly and efficiently remove something from a bag or remove his clothes and put them on [48]. The most useful neurological assessment test for functional leg weakness is Hoover’s sign [51]. It could be performed in two ways: by testing hip extension or hip flexion. The hip flexion test has not been adequately evaluated [48]. Hip extension with 67-100% positive predictive value is practically valid [47] (Figure IA). Other elements of neurological examination motor symptoms in FSM patients are:
Figure I
Positive clinical signs in the neurological examination of functional neurological disorder patients. Red sock – limb with functional weakness [49, 51]
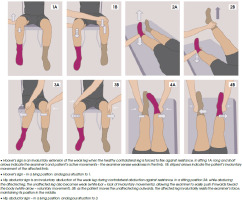
hip abductor sign (Figure IB);
drift without pronation of the functional affected arm;
one-side facial lip pulling/platysma contraction/jaw deviation in case of functional face asymmetry;
give-up or give-way weakness in which normal strength of effort suddenly gives way;
global or inverse pyramidal pattern of weakness, when extensors of the upper limb are weaker than flexors and opposite in the lower limb [47, 52, 53].
Although the positive predictive values of the motor clinical signs typical for FSM are satisfied, there are some pitfalls [54]. The positive Hoover’s sign was reported in the stroke of the supplementary motor area [55, 56]. Thalamic infarction could result in rare cases of the “drift without pronation” sign or isolated “astasia”, imitating motor symptoms inconsistency, which is typical for FSM [57-59].
Functional sensory deficits are often observed together with functional motor deficits. Some proposed diagnostic signs for functional sensory deficits, like non- anatomic distribution of the symptoms or midline splitting, do not have the required specificity. Important pitfalls are “non-anatomical” distribution of sensory deficits reported in small cortical strokes [54, 60].
A wide range of functional speech and language disorders could be observed in FSM patients, such as aphonia, dysphonia, stuttering, dysarthria, or aphasia [47]. FSM patients are approximately ten years younger than those with stroke and seem to be more likely women. Their cerebrovascular risk factors (hypertension, diabetes, dyslipidemia) are lower. On initial presentation, they have lower NIHSS scores [21, 34, 36, 50, 61]. These data were used in several scales proposed over the last few years to differentiate strokes from mimics. Some of the latest propositions are the Telestroke Mimic Score, FABS, and Khan score [62-64]. Telestroke Mimic Score was based on age, medical history (atrial fibrillation, hypertension, seizures), facial weakness and NIHSS score value [62]. FABS is an intuitive scoring system including six variables: absence of facial droop, negative history of atrial fibrillation, age, systolic blood pressure at presentation, history of seizures and isolated sensory deficit without limb weakness at presentation [63]. Khan et al. [63] proposed an algorithm for triaging acute SM, including age, stroke risk factors and other factors like migraines, epilepsy and psychiatric illness [64]. The use of the scores could reduce the rate of the misdiagnosis of the stroke.
The best way to distinguish between FSM and stroke is through clinical bedside evaluation by an experienced neurologist [32]. Even then, physicians agree only to a modest extent; they agree that the most likely final diagnosis of stroke versus SM 71% of the time [65].
Numerous patients may present with different nonvascular neurological conditions that closely resemble a stroke. Except for FSM, these are migraine, seizures, electrolyte or metabolic imbalance, Bell’s palsy, chest pain, collapse, lower respiratory tract infection, previous stroke, vasculitis, glioma, encephalitis, and vestibular neuritis [24, 61, 66, 67]. Patients with FSM are younger than other SM patients [21, 68].
There are some diagnostic pitfalls in FND and, thus, in FSM. Common reasons for a wrong diagnosis include jumping to conclusions based on the patient’s physiological history or stress, failure to consider additional medical or neurological causes, not basing diagnosis on the presence of positive diagnostic signs, diagnosis based on “bizarre presentation” alone and reliance on standard investigations.
On the other hand, a diagnosis of FND could be missed because of the absence of psychological risk factors, insufficient knowledge of FND by the clinician, and placing too much importance on incidental imaging findings. The FND are commonly underdiagnosed in older men [10].
In the discussed case, the middle-aged male represents a group of patients for whom the functional impairments are generally unexpected. The history of multiple vascular incidents without visible changes on neuroimaging suggested the diagnosis of FSM. However, the complete clinical history became available for the medical staff in subsequent days of hospitalisation. This diagnostic clue was highlighted as a red flag in the algorithm (Figure II).
Only a few case reports of FSM has been found in our literature review [69, 70]. Moore et al. [69] discussed a patient with a previous history of TIA and catheterisation with drug-eluting stent placement. In that case, the history of vessel pathology was well documented, which is not the case for the presented patient. The patient reported by Moore et al. [69], did not receive tPA treatment, as she was presented outside the therapeutic window. In the case reported by Segal et al. [70], tPA was administrated. Both reported patients had atypical results from physical examination, and the Hoover’s sign was present in the patient discussed by Segal et al. [70]. In both reported cases, the FSM diagnosis was established not due to the first neurological assessment but after more extended observation in the neurology department. This was also the case for the patient presented in this paper.
Thrombolysis in FSM
Patients with FSM and patients with seizures are most commonly thrombolysed in all SM patients. Among the patients who received ivrtPA for AIS symptoms 4.5% were finally diagnosed as SM. No difference in the frequency of SM thrombolysis between day- and night-time was found in the Canadian study [71]. No adverse events related to administering thrombolysis were reported in this group. Most SM patients were discharged home rapidly with one day of median length of stay [72]. We found only one report on multiple administrations of intravenous thrombolytic therapy to a SM patient [73]. As in our patient, the alteplase was administered several times in different hospitals. Both patients had several cerebrovascular risk factors, which raised the pretest probability of stroke [1, 74]. In the presented patient, the diagnosis of dissociative disorders or simulation was proposed three years before the last presentation to the hospital with typical symptoms. The patient did not inform the hospital staff about that previous diagnosis. The same was reported by Liberman et al. [1]; they postulated the necessity and importance of timely access to electronic health information exchanged among healthcare providers to avoid potential harm to the patient.
Could we improve the assessment of the patient with suspected acute stroke to avoid thrombolytic treatment in case of FSM?
Although thrombolytic treatment seems safe for FSM patients, improvement in their assessment could help avoid ivtPA administration and potential harm. Neuroradiological techniques are standard and widely used methods in suspected acute stroke. The first brain imaging for most patients is non-contrast CT. Very early, it may show no evidence of an acute stroke and is not helpful in the differential diagnosis of the SM [32]. Further CT sequences, including CT-angiography (CTA) and CT perfusion (CTP), can improve the diagnosis. The most sensitive diagnostic tool is DWI-MRI [25]. Buck et al. [32] suggest that additional imaging information should be obtained if SM are possible. First is multimodal CT (non-contrast CT plus CTA/CTP); if the imaging is normal, DWI-MRI should be performed. In patients where suspicion of ischemic stroke is high, and MRI is normal, high-field 3T MRI imaging may show a lesion. The limitation of the assessment is the fact that normal DWI-MRI and MRI fluid-attenuated inversion recovery (MRI-FLAIR) do not formally rule out an AIS in candidates for thrombolysis. Vascular investigations looking for arterial occlusions should be added to avoid withholding effective treatment options in true AIS [49]. Artificial intelligence (AI) could support diagnostic decisions [75].
When neuroimaging, especially DWI-MRI, does not reveal an ischemic lesion in ivtPA-treated patients, the SM diagnosis is most likely, especially when symptoms indicate anterior circulation localisation [32, 76].
Brain perfusion imaging enables the depiction of regional hemodynamic conditions. Even if no standard values are in place for CT perfusion imaging (CTP), Siegler et al. [77] propose that a fully discordant time to a maximum of the residual function (Tmax) > 6 seconds could be a good predictor of SM. MR perfusion (MRP) in AIS patients has some disadvantages compared to CTP. The main ones are limited availability and longer time needed for the performance of MRP. Technological advances should solve these problems in the future [78]. The AI software solutions already include automated post-processing of perfusion data [79]. No abnormalities in brain perfusion imaging support the FSM diagnosis.
Safety of thrombolytic treatment in FSM
The potential harm of thrombolysis for SM patients is a concern for neurologists. Several reports found in the literature have demonstrated the safety of the ivtPA treatment in SM [49, 80] with an intracerebral haemorrhage (ICH) rate ranging from 0 to 1.2%. Even though there are no reports of symptomatic ICH in FSM patients after ivtPA, it remains a potential complication [81]. It should also be considered that physicians could avoid sharing their experiences of incorrect FSM patient qualification for the ivtPA administration, resulting in serious complications or death [49]. Additionally, the ivtPA treatment in FSM patients generates unnecessary costs and improper utilisation of acute stroke care beds [32]. Hospitals with limited experience in stroke thrombolysis (less than 30 cases per year) were more likely to administer ivtPA to SM. There has been an overall trend of increasing thrombolytic treatment for SM in recent years [61].
Tenecteplase (TNK) is a genetically engineered tissue plasminogen activator. Its safety and efficacy were confirmed for reperfusion therapy in AIS [82]. One Norwegian study reported no complications after TNK administration in young patients with SM, but the safety of TNK in mimics has not been definitively established [83].
Long-term treatment and outcome
FSM-diagnosed patients need long-term treatment, starting with an open and clear communication of the problem and a positive diagnosis. A clear explanation helps patients understand the diagnosis. It should be emphasised that the diagnosis is based primarily on specific clinical signs [47]. Jones et al. [7] published a practical scheme of therapeutic intervention, highlighting the critical role of physio- and psychotherapy, especially for persistent symptoms. Their suggested step-care pathway has three steps. First, symptoms are assessed and communicated with information that they are genuine, common, and treatable. Reassuring the patient that there is no structural lesion is recommended [32]. Explaining the importance of positive signs from neurological assessment in the diagnosis is helpful. When possible, they should be demonstrated [7, 84]. Significant risk factors that might indicate the need for higher-intensity treatment should also be identified at that stage. The second step is a low-intensity intervention with more detailed psychoeducation and physiotherapy. The third step is a long-term specialist intervention, which is needed only for a few FSM patients [7]. Patients with serious physical dysfunction may benefit from physiotherapy [85].
The principle of physiotherapy is to promote normal automatic movements and emphasise goal-directed task-specific movements (e.g., kicking a ball). Focusing on goal-directed activities is crucial in occupational therapy. A full-length mirror or video for visual feedback could be useful. Importantly, physiotherapy for FSM patients is different from that for stroke. Techniques focusing on the impairment (strengthening or balancing exercises) should be avoided [42, 47].
Assessment of potential predisposing, precipitating, and perpetuating factors is important in psychological therapy. If acute psychiatric symptoms (severe anxiety or suicidality) are present, a psychiatric assessment is necessary. Comorbid psychiatric disorders (anxiety, depression, etc.) should be treated [47].
Some patients need speech and language therapy with the identification and promotion of normal automatic sounds [47, 86].
Little is known about the long-term outcomes of FSM. Approximately half of the patients with FSM showed no improvement in the long term [47, 87]. In the follow-up, the physical disability was high but could be prevented with early diagnosis. Short duration of symptoms, early diagnosis and high satisfaction with care predicted positive outcomes unlike personality disorders [50, 88].
Perspectives in FSM
Continuous education is needed on FSM diagnostic criteria, specific elements of neurological assessment, and its pitfalls. The broader availability of brain perfusion imaging, both CT and MRI, could result in better detection of SM. The results depend on the perfusion map interpretation, which could challenge the stroke team working under time-pressure [78]. Some promising perspectives are offered by biomarkers which could differentiate ischemic stroke patients from FSM. Kakkar et al. [89] suggest that a combination of NIHSS score and the blood serum level of some blood-brain barrier proteins (occludin, zonula occludens 1, claudin-5) could differentiate between IS and SM.
CONCLUSIONS
FSM should be diagnosed using diagnostic criteria by Hallet et al. [10]. The most common symptoms of FSM are functional weakness and sensory disturbances. Data from medical and personal history, neurological examination and additional test results (neuroimaging) could help confirm the diagnosis. The key finding in the clinical examination is the inconsistency of the symptoms. Treatment with ivtPA appears safe if applied to FSM patients. Patients with FSM need long-term treatment, including physio- and psychotherapy. Long-term prognosis is unfavourable – half of the diagnosed patients showed no improvement.