Purpose
Low-dose-rate (LDR) iodine-125 (125I) brachytherapy is a well-established treatment option for low-risk prostate cancer [1]. Although LDR brachytherapy has been used in Australia as a curative treatment for non-metastatic prostate cancer since 1998 [2], radical prostatectomy still remains the preferred option recommended for younger men due to the unknown long-term outcomes associated with brachytherapy [3]. Recent long-term data has demonstrated that LDR brachytherapy is a treatment option with equivalent or superior long-term control [4]. Clinical guidelines for treatment of prostate cancer recommend brachytherapy as a treatment option either as monotherapy or combined with hormone therapy and/or external beam radiotherapy (EBRT), depending on pre-treatment risk stratification and life expectancy greater than 10 years [3, 5, 6]. However, there has been an overall decline in the use of brachytherapy by radiation oncology communities [7], attributed to the current emphasis on less aggressive treatment strategies, such as the active surveillance, emergence of stereotactic radiation therapy as a convenient alternative, and the need for experienced personnel and specialized equipment to run a brachytherapy service [2]. There has been a recognized increase of the incidence of prostate cancer in men aged 50-59 years since the introduction of prostate specific antigen (PSA) screening in the mid-1980s [8]. Despite the evidence that radiation approaches are appropriate for young patients, radical surgery is favored for this patient population [9]. Given its overall decrease in clinical use, our study aimed to highlight the success of LDR brachytherapy for younger men diagnosed with localized prostate cancer. We reported acute and long-term genitourinary (GU), gastrointestinal (GI), and erectile outcomes in addition to PSA relapse-free survival (RFS), prostate cancer-specific, and overall survival (OS) of patients aged less than 60 years.
Material and methods
This retrospective case series assessed the effectiveness and safety of LDR brachytherapy for all patients attending a private radiation oncology center (GenesisCare Victoria) in Melbourne, Australia, between 2003 and 2016. Institutional review board approval was obtained for the study from the Victoria Research Committee, with approval granted following meeting held on February 6, 2018. All methods were carried out in accordance with committee guidelines of the National Statement on Ethical Conduct in Human Research. Patient consent was included at the time of treatment consent as per GenesisCare Victoria consent process. All patients treated with 125I brachytherapy as monotherapy were included, and their medical history, physical examination, and serum PSA were assessed. T stage was assigned by digital rectal examination. Staging with abdominal/pelvic computerized tomography (CT) and whole-body bone scan was required for patients with Gleason score 7. Pathologists in private pathology laboratories assigned Gleason scores from biopsies.
Patients were stratified according to NCCN classification as follow: low-risk: PSA ≤ 10 ng/ml, Gleason score ≤ 6, and stage cT1-cT2a prostate cancer; intermediate-risk: PSA 10-20 ng/ml, Gleason score 7, and stage cT2b-c prostate cancer. Favorable was defined as 1 risk factor, Gleason 3 + 4 = 7, and < 50% core biopsy positivity. Unfavorable was defined as 2 or 3 risk factors, Gleason 4 + 3 = 7, and/or ≥ 50% core positivity. In cases where T2 sub-stage was not specified but Gleason score was 6 and PSA ≤ 10, patients were assigned as stage cT1c and classified as ‘low-risk’.
This study implemented the same volume study, treatment planning, and treatment techniques as previously reported by Chao et al. [2]. Dosimetric parameters were consistent with the European Society for Radiotherapy and Oncology (ESTRO) recommendations for prostate D90, dose received by 90% of the prostate, V100 and V150, percentage of the prostate receiving 100% and 150% of prescription dose, respectively, along with dose constraints recommended for the rectum and urethra [10].
Patients were followed up by a radiation oncologist (RO) for one month after LDR brachytherapy, then every 6 months with a repeated PSA, and yearly thereafter. In addition, patients agreed to PSA testing for a minimum of four years. Biochemical progression-free survival (bPFS), clinical progression-free survival (cPFS), overall survival (OS), and other associated treatment toxicities were recorded. bPFS was defined with Phoenix definition of nadir +2 ng/l, from the date of implant to the date of first PSA meeting the criteria and excluding any PSA bounce [11]. cPFS was defined as the presence of imaging detecting local, regional, or distant disease from the date of implant to the date of clinical investigation. OS time was measured from the date of implant to the date of death from any cause, and censored for those alive at the date of last contact or closeout date, whichever was earlier. PSA bounce was defined as a short-term increase of > 0.2 ng/l in PSA level, occurring more than 3 months after implantation and followed by a spontaneous decline without intervention [12]. Date of the bounce was recorded at the highest PSA value, and the height of the bounce was measured from the lowest PSA value between the implant and bounce. Late gastrointestinal (GI) and genitourinary (GU) toxicities reported 90 days after implantation were graded according to the common terminology criteria for adverse events version 4.0 (CTCAE v. 4). The analysis was based on the evaluation of maximum toxicity score throughout treatment for each patient. Erectile dysfunction was defined as erection not capable of penetration, or no erection in a patient who was previously potent.
Descriptive statistics were prepared to characterize the patient, disease, and treatment features as well as toxicities after treatment. Results were presented as mean and standard deviation (SD) or median (interquartile range [IQR] or overall range) depending on the underlying distribution of data. In case of the counts, crude numbers and/ or percentages were presented. All time-to-event points were measured from the date of implant to the date of last PSA test, with censoring considered from the date of relapse. Proportional hazards Cox regression models were used to assess differences in time to event outcomes across patient characteristics, and presented as hazard ratios (HR) with 95% confidence intervals and charted according to Kaplan-Meier method. Statistical analysis was conducted using Stata version 15.1 (StataCorp, College Station, Texas, USA), with p-value of less than 0.05 considered statistically significant. Dataset used and analyzed during the current study is available from the corresponding author on reasonable request.
Results
Patient characteristics
Over the study period, 161 patients met the inclusion criteria, with a mean age at implant of 56 years (SD = 3.97). The mean PSA level at diagnosis was 4.43 ng/ml (SD = 2.29). The staging was T1 in 44.7% and T2 in 55.3% of the patients. 65 patients (40.3%) underwent trans-urethral resection of the prostate (TURP) prior to implant. Table 1 shows the details of patient characteristics. At the time of treatment, 70.2% of patients were classified as having low-risk disease, with 29.8% of patients having intermediate-risk disease.
Table 1
Patient characteristics, n (%) unless otherwise indicated
Time to relapse-free survival
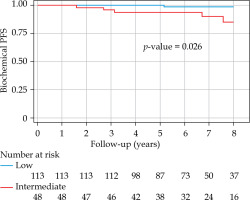
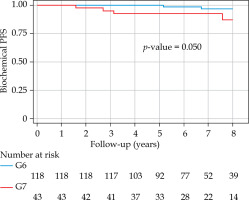
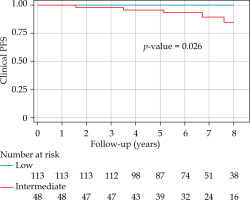
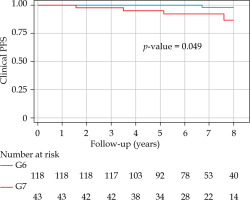
With a median follow-up of 6.8 years (minimum, 3 years; maximum, 14.54 years), bPFS at 8 years was 94% (95% CI: 87-98%) for the entire cohort, with 99% and 85% for low- and intermediate-risk groups, respectively (Figure 1). The bPFS at 8 years follow-up was better for Gleason 6 disease (96%; 95% CI: 89-99%) compared with Gleason 7 disease (88%; 95% CI: 67-96%) (Figure 2). The cPFS at 8 years for the entire cohort was 95% (95% CI: 88-98%); low- and intermediate-risk groups were 100% and 85%, respectively (Figure 3). The cPFS at 8 years follow-up was better for Gleason 6 disease (98%) compared with Gleason 7 disease (87%) (Figure 4). Overall, 6 patients had clinical progression of their disease. Four patients experienced local recurrence: three underwent salvage prostatectomy, while one declined surgery and was considered lost to follow-up. Of the three patients who underwent prostatectomy, one died from distant metastases, one was started on ADT for PSA progression, and one had an undetectable PSA. Two other patients were started on hormonal therapy following nodal/distant metastases.
Overall survival
Overall survival was 96.9% for the entire cohort. Five deaths were reported. Two patients died following acute myocardial infarctions, one died from an unknown cause, one died from metastatic bladder cancer, and only one death was secondary to prostate cancer. The prostate cancer specific survival (PCSS) rate was 99.4%.
PSA bounce
Thirty-seven percent of patients had a PSA bounce within 3 years of implant, with a median of 0.72 mg/ml (interquartile range, 0.34-1.34 mg/ml; range, 0.2-4.52 mg/ml) and a median duration of 0.52 years (interquartile range, 0.36-0.58 years; range, 0.09-1.69 years). All patients had at least 3 years of follow-up to minimize the impact of PSA bounce when analyzing bRFS. At 4 years follow-up, 140 of the 161 patients had PSA readings. The median PSA at 4 years was 0.169 (IQR, 0.096-0.360), with 45% of patients having a PSA greater than 0.2.
Late toxicities
Late grade ≥ 3 genitourinary toxicities were reported in 18 patients (11.2%), with only one patient (0.6%) experiencing late grade 3 urinary incontinence after salvage radical prostatectomy (Table 2). All 12 patients with late grade 3 urinary retention (urethral strictures) had either follow-up urethral dilatation (n = 5), TURP (n = 5), or mini-TURP (n = 2), with resolution of their symptoms. Another 6 patients reported late grade 3 hematuria either from the prostatic fossa or bladder requiring surgical intervention. One patient reported both late grade 3 urinary retention and hematuria. Twelve patients developed late grade 2 urinary retention, another 3 developed grade 2 hematuria, and one patient developed both urinary retention and hematuria. No patients who underwent TURP prior to brachytherapy reported any late grade 3 GU toxicity. Nineteen patients (11.8%) experienced late grade 1-2 gastrointestinal (GI) toxicity. The incidence of erectile dysfunction was 21.7%.
Table 2
Late toxicities. Data presented as number and percentage
Secondary malignancies
In the current study, three patients (1.9%) developed secondary cancers, all considered unrelated to their LDR brachytherapy. These included one patient, who developed renal cell carcinoma, one patient, who developed advanced colon cancer, and one patient, who developed poorly differentiated signet ring cell carcinoma of the bladder.
Discussion
The purpose of this study was to evaluate the treatment outcomes of men under 60 years of age, who underwent LDR brachytherapy with 125I for clinically localized low- to intermediate-risk prostate cancer. The bRFS at 8 years post-LDR implant was 94% for the entire cohort, and OS was 96.9%. Our present results support several other studies investigating prostate brachytherapy in men aged ≤ 60 years [3, 13, 14].
Langley et al. [3] reported outcomes of 597 patients aged ≤ 60 years, who received brachytherapy with or without EBRT and/or hormonal therapy. Of their cohort, 7.4% had treatment failures, with a median time to recurrence of 5.5 years. Six patients died from prostate cancer at a median of 6.4 years after implantation, and seven died from other causes after a median of 5.9 years from treatment. Similarly, Kollmeier et al. [14] described outcomes of 236 patients aged ≤ 60 years upon receiving LDR brachytherapy or high-dose-rate brachytherapy (11% received high-dose-rate brachytherapy). The reported 8-year OS and PSA relapse-free survival (RFS) rates were 96% and 96%, respectively. In the present study, the 8-year OS, PCSS, and bRFS rates were 96.9%, 99.4%, and 94.0%, respectively. In Kollmeier et al. study, for patients with low- and intermediate-risk disease, the 8-year RFS rates were 97% and 94%, respectively, whereas in our study, for patients with low- and intermediate-risk disease, the rates at 8 years were 99% and 85%, respectively.
Ashamalla et al. [15] reported on approximately 16,000 men in the surveillance, epidemiology, and end results (SEER) database, who were aged ≤ 60 years at the time of LDR brachytherapy (with or without EBRT) or EBRT alone. The 8-year overall prostate cancer-specific mortality was 1.9%, and was lower for patients treated with LDR brachytherapy compared with those treated with EBRT alone (1.1% vs. 2.8%, respectively). These results agree with our 8-year PCSS rate of 99.4% as well as the 8-year PCSS rate of 99% reported by Langley et al. and Kollmeier et al. [3, 14].
Toxicity outcomes
Urinary retention was the most common late GU toxicity, with 12 (7.5%) and 13 (8.1%) patients developing grade 2 and 3 urinary retention, respectively. Langley et al. reported a lower risk of grade 2 urinary retention (stenosis/stricture) with 3.2% [3]. Only one patient in their study reported grade 3 treatment-related urinary retention.
Our study found that erectile dysfunction affected 21.7% of our cohort ,which is similar to the results of Langley et al., where 70-80% of brachytherapy monotherapy patients had preserved potency from 3 months to 5 years after implantation [3]. Our results showed less erectile dysfunction compared with Cesaretti et al. [16] (64% at a follow-up of ≥ 7 years) and Buckstein et al. [13], where 69% of patients were potent at 10 years.
Second primary cancers
Although rare, the development of second primary cancers within the radiation field has been recognized as a possible side effect of EBRT [17]. Radiation-induced second primary cancers (SPCs) are tumors that develop later than 5 years after radiation therapy in an irradiated field, with histopathological features different from the primary tumor [18]. Given that prostate cancer is being diagnosed at an earlier age than in the past, long-term consequences, such as SPCs are important to consider [18]. Moon et al. highlighted in 2006 that patients, who received EBRT had significantly higher odds of developing second cancers both overall and in the areas exposed to radiation, emphasizing that patients, who received radioactive implants had the lowest odds of developing second cancers [19]. These findings were further supported by a systematic review and meta-analysis of observational studies published in 2016 describing increased odds for SPCs with EBRT but not as much as with brachytherapy [20]. Specifically, brachytherapy was associated with an increased odds for bladder cancer compared with surgery, but this risk did not apply to other tumor sites [20]. In our study, three patients (1.9%) developed secondary cancers, all considered unrelated to their LDR brachytherapy. These included two patients, who developed renal cell carcinoma and one patient, who developed advanced colon cancer. Interestingly, one patient developed poorly differentiated signet ring cell carcinoma of the bladder. This is a relatively rare histological variant of carcinoma of the urinary bladder, accounting for 0.5-2% of primary tumors of the bladder [21]. This patient had brachytherapy for Gleason 6 prostate cancer in 2009 and developed signet ring cell carcinoma of the bladder in the dome, well away from the bladder base and trigone in 2018. This patient was treated with cystoprostatectomy and adjuvant chemotherapy, but unfortunately succumbed to his aggressive bladder cancer.
Limitations
Study limitations include the retrospective nature of our dataset, although median follow-up of 6.8 years provides confidence in our ability to ascertain cancer control and toxicity. Like Langley et al. suggested in their study [3], further follow-up is required to capture true incidence of SPCs in our cohort. Our study was also limited by a relatively small number of patients studied. The results of our study are similar to other regionally-based published research that have demonstrated the benefit of LDR brachytherapy for men aged ≤ 60 years with clinically localized, low- to intermediate-risk prostate cancer. Our findings suggest that brachytherapy is as effective as other modalities of radiation and surgery with comparable PCSS. With excellent long-term treatment outcomes and minimal associated toxicities, our results showed that LDR brachytherapy is also an effective treatment option for this younger cohort of men diagnosed with localized prostate cancer.