INTRODUCTION
Therapy of nivolumab and other immune checkpoint inhibition drugs are one of the fastest growing fields of oncology. They use antibodies in order to boost personal immune response, which is altered by cancer cells. During the last decade physicians have been observing a rising problem of adverse effects after treatment with immune checkpoint inhibitors.
It was reported that up to 40% [1] of patients developed immune-related adverse effects during therapy. They ranged from mild to life-threatening reactions and involved multiple systems, e.g. respiratory, gastrointestinal, endocrine system.
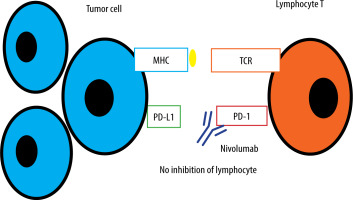
Nivolumab is a PD-1-blocking monoclonal human antibody which was registered in 2014 [2]. Checkpoint inhibitor drugs assist the immune system in eliminating malignant cells. To work properly lymphocyte T needs a stimulatory signal – between the T cell receptor on the lymphocyte and the major histocompatibility complex molecule on an antigen-presenting cell. In order to trigger T cells, absence of inhibitory signals is also required. Cancer cells induce tolerance among T cells by expressing ligands (for example PD-L1) that bind to inhibitory receptors on T cells [3]. This stops the local immune response and allows tumour to grow uninterrupted. Nivolumab through the PD-1 receptor pathway causes the immune checkpoint blockade because of diminished inhibitory signalling (Figure 1).
CASE REPORT
A 66-year-old man with metastatic melanoma of the scalp, small intestine neuroendocrine tumour, pulmonary berylliosis, type 2 diabetes mellitus, hypertension and hyperthyroidism was admitted to the Pulmonology and Allergology Department because of exertional dyspnoea and suspected interstitial lung disease.
The patient was diagnosed with small intestine neuroendocrine tumour and underwent surgery in 2018. Melanoma with cervical lymph node metastases was detected in 2019. Firstly, the patient had surgical resection of melanoma and metastatic lesions. Because of progression of the disease he started treatment with nivolumab in June 2020. He received 480 mg of nivolumab intravenously every month. On follow-up high-resolution computed tomography (HRCT) at the Oncology Clinic in December 2021, discrete subpleural interstitial lung abnormalities were shown. A control HRCT was conducted 3 months later and demonstrated significant progression of interstitial changes. Because of abnormalities in HRCT, nivolumab administration was stopped in February 2022. The patient received in total 23 nivolumab infusions.
On admission he was in good general condition with blood pressure of 108/75 mm Hg, oxygen saturation on atmospheric air of 95%, regular heart rate of 80/min. On physical examination, crackles were heard at the lung bases bilaterally. Laboratory blood tests revealed mild normocytic, normochromic anaemia and slightly increased levels of C-reactive protein (CRP) of 21.30 mg/l without any other evidence of infection. HRCT scan, 2 months after the last HRCT at the Oncology Clinic, was performed on admission and revealed aggravation of interstitial changes, reticular and small nodular changes with predominantly supradiaphragmatic distribution in bilateral lung fields and bronchiectasis in the lower and middle right lobe. Pulmonary function tests were conducted: spirometry and bronchodilator test (results within normal range) and diffusing capacity of the lungs for carbon monoxide (DLCO) (mild reduction, 69% to lower limit of normal).
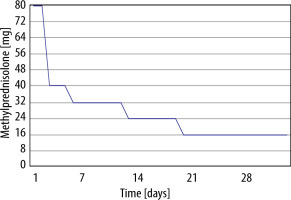
The patient underwent a bronchoscopy which showed a small amount of mucous secretion. The bronchial lavage fluid was collected for examination. Physiological bacterial flora of the respiratory tract was cultured from the collected material. The bronchoalveolar lavage fluid (BALF) showed dominant lymphocytes (62.5%). With the exclusion of an infectious cause, he was diagnosed with nivolumab-induced interstitial lung disease. He received methylprednisolone (MP) intravenously at a dose of 80 mg/day for 2 days; MP dose was reduced to 40 mg/day daily for 3 days. The patient was discharged with recommendations to gradually taper the MP dose to 16 mg/day and to discontinue nivolumab therapy (Figure 2).
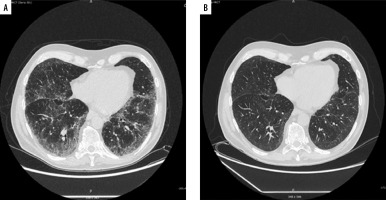
After 1 month of treatment, he was readmitted to hospital to assess response to steroid therapy. He felt better, he did not report exertional dyspnoea. On auscultation, normal lung sounds were heard. There was an improvement in DLCO (increase from 69% to 80% of predicted). A follow-up chest HRCT scan showed significant regression of interstitial changes (Figure 3). Further gradual reduction of oral steroids and follow-up in the Pulmonology Outpatient Clinic were recommended. After 5 months of slow dose reduction (from 16 mg to 2 mg daily), methylprednisolone treatment was discontinued. During follow-up visits in the Pulmonology Outpatient Clinic, a control chest HRCT after 6 months was ordered. In computed tomography further regression of interstitial changes was described, and the patient reported no symptoms.
Figure 3
High-resolution computed tomography. A – After 23 doses of nivolumab, without treatment with methylprednisolone, B – after 1 month of methylprednisolone therapy
Nivolumab is a PD-1-blocking monoclonal human antibody, first registered in 2014 [2]. Checkpoint inhibitor drugs assist the immune system in eliminating malignant cells. To work properly lymphocyte T needs a stimulatory signal – between the T cell receptor on the lymphocyte and the major histocompatibility complex molecule on an antigen-presenting cell. In order to trigger T cells, absence of inhibitory signals is also required. Cancer cells induce tolerance among T cells by expressing ligands (for example PD-L1) that bind to inhibitory receptors on T cells [3]. This stops the local immune response and allows tumour to grow uninterrupted. Nivolumab blocks the PD-1 receptor, thus eliminating the inhibitory signal. The idea of immunotherapy, although effective and brilliant, brings some disadvantages to patients. Immune-related adverse effects are an increasing problem which sometimes leads to discontinuation of therapy. When it comes to severe side effects, 21% of patients receiving nivolumab and 59% of people on a combination of anti-CTLA4 antibodies and nivolumab [4] suffer from them.
Although research is still in progress, the main culprit seems to be impaired self-tolerance from loss of T-cell inhibition [5]. Among healthy individuals immune checkpoint proteins provide maintaining peripheral tolerance to self-tissues. When they are overly blocked, many autoimmune-related illnesses can occur.
The presented patient developed drug-induced interstitial lung disease (DIILD). In 2022 the European Society for Medical Oncology (ESMO) published guidelines [6] on diagnosis and treatment of DIILD. ESMO proposed a new classification of managing immunotherapy-related toxicity which divides symptoms into 4 grades [6]. Grade 1 drug-induced interstitial lung disease (DIILD) is defined by the absence of respiratory symptoms, with radiographic findings only. Grade 2 DIILD is defined by the onset of mild respiratory symptoms that do not negatively impact the patient’s quality of life. Grade 3 (severe) DIILD, however, is defined by the occurrence of symptoms that lead to a worsening of the patient’s quality of life and limit their activities of daily living, including the possible need for oxygen therapy, regardless of the radiological severity. Grade 4 (very severe, life-threatening or disabling) DIILD is defined as the occurrence of severe, disabling symptoms leading to hospitalisation and possibly mechanical ventilatory support. In this case, negative bacterial, viral and mycology tests and elevated lymphocyte number in BALF were important steps of differential diagnosis. Developing symptoms after almost 2 years of using drug is uncommon, but possible. The patient’s disease severity was classified as grade 2. He presented with mild exertional dyspnoea not observed earlier and basal crackles. ESMO recommends immediate discontinuation of the anticancer drug and starting 1–2 mg/kg/day steroid therapy with prednisone or equivalent tapering over 4–6 weeks. In this case, because of mild symptoms, tapering the dose of steroids started early. The most concerning part was extensive and progressing radiological findings. After 1 month of steroid therapy, as mentioned before, a significant improvement in chest HRCT and symptoms was observed. With grade 2 symptoms, which are mild and do not negatively impact the patient’s quality of life, it is possible to continue anticancer therapy in some individuals after the symptoms disappeared. Such a decision must be preceded by oncological assessment of the risks and benefits. Safety of rechallenging needs more research. A retrospective analysis of 40 rechallenged patients with anti-PD-(L)1 therapy found that 42.5% developed recurrences of prior adverse effects and that 12.5% developed new symptoms from other organs [7]. In this patient oncological assessment was performed and the therapy was permanently discontinued. In the future, the number of patients with drug-induced lung diseases will probably increase due to invention of new drugs and growing popularity of this treatment. In most cases, the course of steroid therapy and discontinuation of the culprit drug help to resolve symptoms. However, rarely, symptoms can be life-threatening.
CONCLUSIONS
This case report reminds how important careful follow-up during checkpoint inhibitor therapy is. Another valuable information is unusual time of onset of DIILD. Median time to onset of DIILD is 2.8 months [8], compared to almost 2 years in this patient. It shows that immune-related side effects can occur after a long time of complication-free therapy. Most of immune-related adverse events during nivolumab therapy are mild and can be successfully treated with steroids. The key is constant alertness for many possible adverse effects from multiple organs.