Introduction
Acid-related diseases, also known as peptic-acid diseases, result from distinct but overlapping pathogenic mechanisms that involve acid effects on an oesophagogastric duodenal mucosa with diminished defence. Gastroesophageal reflux disease (GERD) – especially that leading to esophagitis, peptic ulcers, and ulcers caused by the use of non-steroidal anti-inflammatory drugs (NSAIDs) and acetylsalicylic acid (ASA) – are some of the conditions that need to be highlighted [1, 2].
GERD represents the most frequently observed acid-related disease [1]. Globally, the mean prevalence in 2017 ranged from 4408 cases per 100,000 to 14,035 cases per 100,000 population, and countries from Latin America were among those with the highest values (> 11,000 cases per 100,000) [3]. In Brazil, estimates of the frequency of GERD or GERD symptoms such as heartburn ranged from 7.3% to 20.8% [4, 5]. In addition, 6.01 million years lived with disability were estimated considering only GERD in 2017, pointing to the relevant impact of acid-related diseases [3]. Regarding the occurrence of a peptic ulcer due to NSAID use, a study conducted in Brazil reported the association of the use of these medications and the presence of gastric lesions on endoscopy, with an increase in the risk of having gastric ulcer, gastric erosion with hematin, and gastric erosion of 2.17 (95% CI: 0.83–5.70), 2.91 (95% CI: 1.27–6.71), and 2.21 (95% CI: 1.50–3.27)-fold, respectively [6].
Treatment goals are related to symptoms relief, ulcer healing, and prevention of complications and recurrence [1]. Acid-related disease treatment is based on proton pump inhibitors (PPIs) worldwide [7–10]. In Brazil, PPIs have been the gold-standard treatment strategy for GERD management since the 1980s [11].
However, despite the efficiency of PPIs, several limitations lead to unmet medical needs in acid-related disease management, such as delay in the onset of the effect, low bioavailability, fast metabolism, drug interactions, variable sustainability of acid suppression, enteric-coated pharmaceutical form, and nocturnal acidity breakthrough [12]. In addition, it is estimated that about 6–15% and 40–50% of patients with non-erosive reflux disease and erosive oesophagitis, respectively, will fail to respond to treatment with PPIs [13]. This is especially true among patients with severe erosive disease (Los Angeles classifications C and D), in which failure rates are estimated at 20–30% [13]. The global failure of PPI therapy results in persistence of symptoms, occurrence of new symptoms, and relapse of healed oesophagitis during maintenance therapy.
In this context, a new therapeutic class, potassium-competitive acid blockers (P-CABs), has emerged to promote a superior antisecretory effect addressing these unmet needs related to acid-related disease management [14]. Pharmaceutical companies have proposed several drugs from the P-CABs class; however, safety issues have led to discontinuation of most of them. Vonoprazan fumarate and revaprazan showed better safety profiles and were the first drugs of the P-CABs class to be approved by regulatory agencies [15, 16]. Vonoprazan is a new molecule recently approved by the Brazilian National Health Surveillance Agency (ANVISA, Agencia Nacional de Vigilancia Sanitaria), which could bring more answers for acid-related diseases’ unmet needs in the country [17].
Aim
This narrative review was conducted to provide an overview of the general concepts regarding P-CABs, focussing on vonoprazan fumarate.
Material and methods
A literature search was conducted through April–May 2021, using PubMed, LILACS (Literatura Latino-Americana e do Caribe em Ciencias da Saude), and the Scielo databases, using terms related to acid-related diseases and P-CABs. Non-structured searches for efficacy and safety information were performed using a combination of MeSH controlled vocabulary and text words such as “vonoprazan”, “1-(5-(2-fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl)-N-methylmethanamine”, “TAK 438”, and “YH 1885”. Papers that described pivotal and novel insights about P-CABs and vonoprazan fumarate were selected. Additional studies were also found using the bibliographies of selected articles.
Historical evolution of treatment of acid-related diseases’
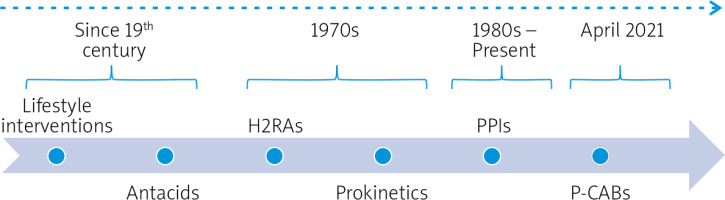
Lifestyle interventions were the first strategy to be proposed based on the assumption that obesity, smoking, alcohol consumption, body position, and food intake could prevent the retrograde flow of gastric contents. Later, acid inhibitors were developed to treat GERD by inhibiting acid secretionconsidering that acid exposure could explain acid-related diseases. Antacids were initially proposed based on the association of the condition with an excessive amount of gastric acid; other medications include histamine H2-receptor antagonists (H2RAS), prokinetics, and PPIs [11]. The use of PPIs emerged from the observation of the gastric H+/K+-ATPase on the proton pump as the final step of the acid secretion pathway, turning into the preferred treatment option. However, unmet medical needs related to insufficient gastric acid suppression due to pharmacological limitations are still observed [18]. In the early 1980s, the first P-CAB was developed considering the rationale that this substance could inhibit H+,K+-ATPase in a reversible and K+-competitive manner, which turned into an almost complete gastric acid secretion inhibition since the first dose [15, 18]. The synthesis of a series of N-(5-aryl-1-arylsulfonyl-1H-pyrrole-3-yl) methyl-N-methylmethanamine derivatives resulted in the finding of a compound with potent H+,K+-ATPase inhibition in vitro, and it was selected to be a drug candidate [19]. This compound was an object of further studies, and it is known as vonoprazan fumarate [16].
Recently, ANVISA approved P-CABs vonoprazan, as shown in Figure 1, for GERD management and for the treatment of other acid-related diseases [17]; in Brazil there is no regulatory indication for H. pylori eradication, but vonoprazan-based triple therapy (vonoprazan, amoxicillin, and clarithromycin) or double therapy with 4 g of amoxicillin both for 7 days has shown an eradication rate of approximately 90% [20].
Figure 1
Evolution of GERD treatment in Brazil. Modified from Zaterka et al. (2019) [11]
H2RAs – histamine H2-receptor antagonists, P-CABs – potassium-competitive acid blockers, PPI – proton pump inhibitors.
P-CABs: historical evolution
The class of P-CABs was first described in the 1980s. SCH28080, an imidazopyridine compound, was developed and showed inhibition of gastric acid secretion in humans and animals. In addition, the compound was shown to inhibit H+,K+-ATPase via competitive interaction with the K+ site of the enzyme. However, clinical studies were discontinued due to hepatic toxicity after prolonged administration. Then, a series of studies using SCH28080 derivatives was initiated [15, 21]. Compounds such as linazapran (imidazopyridine derivative), sorazapran (imidazonaphthyridine derivative), SPI-447 (imidazothienopyridine), SK&F96067 and SK&F97574 (quinolone derivative), CS-526 (pyrrolopyridazine derivative), revaprazan (pyrimidine derivative), and vonoprazan (pyrrole derivative) were developed [15].
Two P-CABs are available for use in clinical practice, and they are indicated to manage acid-related diseases. Revaprazan was the first P-CAB used, initially launched in South Korea to treat duodenal ulcer, gastric ulcer, and gastritis, and now also available in India. Vonoprazan was launched in Japan in 2015 to be used for the treatment of gastric ulcer, duodenal ulcer, erosive oesophagitis, and prevention of low-dose aspirin- or NSAID-induced ulcer recurrence [15, 21]. In 2019 it was approved in China for the management of gastric/duodenal ulcer and reflux oesophagitis [22].
P-CABs: mechanism of action
P-CABs are characterized as weak bases, and their protonated form can block the K+ exchange channel of H+, K+-ATPase in the proton pump. The pKa is an important marker: the lower the pKa value, the stronger the acid. The pKa of these drugs varies between 5.6 (SCH28080), 6.1 (linaprazan), and 9.3 (vonoprazan). Considering that vonoprazan has a high pKa (at 9.3), most of it is protonated easily and exerts its inhibitory action. Additionally, because the protonated forms are less prone to cross membranes than the non-ionic molecules, these protonated forms of P-CABs concentrate in the acid-secreting canaliculi of parietal cells where inhibit H+, K+-ATPase enzyme [21, 23].
One disadvantage of PPIs was the acidic parietal cell pH requirement to facilitate the conversion of the prodrug to its active form for the pharmacological effect. On the other hand, the K+ competitive acid blockers do not depend on acid activation but bind to the enzyme directly with a rapid onset action and better control of acid secretion [21, 23]. P-CABs block the K+ exchange channel of the proton pump, resulting in fast, competitive, reversible inhibition of acid secretion [23]. However, because they bind reversibly and not covalently, a constant plasma concentration of the drug has to be maintained for sustained effect. As a result, as shown in Figure 2, they may have a longer duration of action than the PPIs with extended release formulation, which is dependent on the drug half-life [23, 24]. Vonoprazan showed rapid absorption and a mean elimination half-life up to 9 h, with no apparent time-dependent inhibition of metabolism [25].
Figure 2
Illustrated mechanism of action of PPIs as compared with P-CABs. PPIs convert to their active form in acid milieu within the secretory canaliculi and bind covalently to H+/K+-ATPases in a stimulated parietal cell. While P-CABs accumulate in a high concentration in the secretory canaliculi, bind reversibly to H+/K+-ATPases with no need for an acid milieu for drug activation. Modified from Shibli et al. 2020 [24]
H2 – histamine, Ach –acetylcholine, G – gastrin
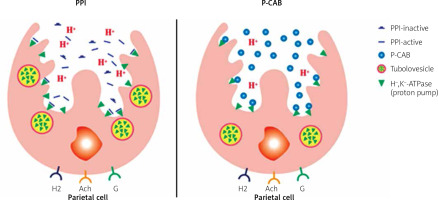
Table I shows the main characteristics responsible for P-CABs’ and PPIs’ mechanism of action differentiation.
Table I
Characteristics and main differences in the mechanism of action of potassium-competitive acid blockers and proton pump inhibitors. Modified from Scarpignato and Hunt (2019) [23]
Vonoprazan: efficacy and safety data
Efficacy and safety of vonoprazan for the management of oesophagitis related to GERD and among patients with low-dose aspirin- or NSAID-induced ulcer were previously assessed in several studies [26–39]. Considering the comparison with PPIs, only studies assessing vonoprazan performance against lansoprazole, which is a potent PPI, were conducted. The primary efficacy outcomes are those related to healing and maintenance. The studies report, in the majority, non-inferiority of vonoprazan to reach such treatment goals in a follow-up period up to 104 weeks [34–39]. Most of the single-arm studies were performed among patients refractory to PPIs, and the results show maintenance rates greater than 80% in a follow-up of up to 52 weeks [28, 29, 31, 32]. Table II shows a summary of the efficacy findings in studies using vonoprazan as a single arm or compared to PPIs.
Table II
Summary of studies assessing vonoprazan efficacy profile
| Author, year | Region | Comparator | Healing outcomes | Maintenance outcomes |
|---|---|---|---|---|
| Ochiai, 2021 [33] | Japan | PPI | Mucosal healing: • Vonoprazan: 68.8% • PPI: 7.1% | NA |
| Xiao, 2020 [40] | Asia | Lansoprazole | Erosive oesophagitis healing rate at 8 weeks: • Vonoprazan 20 mg: 92.4% • Lansoprazole 30 mg: 91.3% Erosive oesophagitis healing rate at 2 weeks: • Vonoprazan 20 mg: 75% • Lansoprazole 30 mg: 67.8% Erosive oesophagitis healing rate at 4 weeks: • Vonoprazan 20 mg: 85.3% • Lansoprazole 30 mg: 83.5% | NA |
| Mizuno, 2019 [28] | Japan | None (single arm) | NA | Non-relapse rate at 48 weeks: • Vonoprazan 10 mg: 86% |
| Tanabe, 2019 [31] | Japan | None (single arm) | NA | Non-relapse rate at 52 weeks: • Vonoprazan 10 mg: 93.8% |
| Ashida, 2018 [36] | Japan | Lansoprazole | NA | Recurrence rate at 24 weeks: • Lansoprazole 15 mg: 16.8% • Vonoprazan 10 mg: 5.1% • Vonoprazan 20 mg: 2.0% Recurrence rate at 12 weeks: • Lansoprazole 15 mg: 12.2% • Vonoprazan 10 mg: 2.5% • Vonoprazan 20 mg: 1.0% |
| Kawai, 2018 [41] | Japan | Lansoprazole | NA | Recurrence rate at 24 weeks: • Lansoprazole 15 mg: 2.8% • Vonoprazan 10 mg: 0.5% • Vonoprazan 20 mg: 1.5% Recurrence rate at 12 weeks: • Lansoprazole 15 mg: 0.9% • Vonoprazan 10 mg: 0.5% • Vonoprazan 20 mg: 0.5% |
| Mizokami, 2018 [35] | Japan | Lansoprazole | NA | Recurrence rate at 12 weeks: • Lansoprazole 15 mg: 5.0% • Vonoprazan 10 mg: 2.9% • Vonoprazan 20 mg: 3.0% Recurrence rate at 24 weeks: • Lansoprazole 15 mg: 5.5% • Vonoprazan 10 mg: 3.3% • Vonoprazan 20 mg: 3.4% Recurrence rate at 52 weeks: • Lansoprazole 15 mg: 7.0% • Vonoprazan 10 mg: 3.8% • Vonoprazan 20 mg: 5.4% Recurrence rate at 76 weeks: • Lansoprazole 15 mg: 7.5% • Vonoprazan 10 mg: 3.8% • Vonoprazan 20 mg: 5.9% Recurrence rate at 104 weeks: • Lansoprazole 15 mg: 7.5% • Vonoprazan 10 mg: 3.8% • Vonoprazan 20 mg: 5.9% |
| Mizuno, 2018 [29] | Japan | None (single arm) | NA | Non-relapse rate at 24 weeks: • Vonoprazan 10 mg: 86% |
| Umezawa, 2018 [30] | Japan | None (single arm) | NA | Non-relapse rate at 24 weeks: • Vonoprazan 10 mg: 86.2% |
| Hoshino, 2017 [32] | Japan | None (single arm) | Erosive oesophagitis healing rate at 4 weeks: • Vonoprazan 20 mg: 87.5% | NA |
| Ashida, 2016 [38] | Japan | Lansoprazole | Erosive oesophagitis healing rate at 8 weeks: • Vonoprazan 20 mg: 99.0% • Lansoprazole 30 mg: 95.5% Erosive oesophagitis healing rate at 2 weeks: • Vonoprazan 20 mg: 90.7% • Lansoprazole 30 mg: 81.9% Erosive oesophagitis healing rate at 4 weeks: • Vonoprazan 20 mg: 96.6% • Lansoprazole 30 mg: 92.5% | NA |
| Ashida, 2015 [39] | Japan | Lansoprazole | Erosive oesophagitis healing rate at 4 weeks: • Vonoprazan 5 mg: 92.3% • Vonoprazan 10 mg: 92.5% • Vonoprazan 20 mg: 94.4% • Vonoprazan 40 mg: 97.0% • Lansoprazole 30 mg: 93.2% Erosive oesophagitis healing rate at 2 weeks: • Vonoprazan 5 mg: 86.0% • Vonoprazan 10 mg: 93.2% • Vonoprazan 20 mg: 93.8% • Vonoprazan 40 mg: 94.8% • Lansoprazole 30 mg: 88.6% Erosive oesophagitis healing rate at 8 weeks: • Vonoprazan 5 mg: 96.5% • Vonoprazan 10 mg: 95.5% • Vonoprazan 20 mg: 96.5% • Vonoprazan 40 mg: 97.0% • Lansoprazole 30 mg: 95.5% | NA |
A randomized controlled trial showed how rapidly vonoprazan (20 mg) and lansoprazole (30 mg) could provide heartburn relief among patients with erosive oesophagitis [37]. The authors reported that the symptom was relieved significantly sooner with vonoprazan, highlighting the differences between P-CABs and PPIs previously described in this review [37].
The pattern of frequency of adverse events is similar to that of PPIs [35, 36, 38–41]. Gastrointestinal disorders (diarrhoea, abdominal distention) are the adverse events most frequently reported by system organ class and are similar in vonoprazan (18.4%) or PPI (19.1%) treatment [40]. However, the frequency of liver function abnormalities, one of the major concerns regarding P-CABs development, was low compared to lansoprazole (0% vs. 0.9%). Vonoprazan is considered safe and well-tolerated, the only concern is the slight increase in gastric endocrine cells [38]. Severe drug-related adverse events were not reported with vonoprazan treatment [40]. Table III shows the results regarding vonoprazan safety profile.
Table III
Summary of studies assessing vonoprazan safety profile
| Author, year | Country | Adverse events – vonoprazan group | Adverse events – comparator group | Adverse events both groups (> 10%) |
|---|---|---|---|---|
| Xiao, 2020 [40] | Asia | TEAEs: 38.1% Leading to discontinuation: 2.0% Liver function abnormalities: 0% SAEs: 1.2% Deaths: 0% | TEAEs: 36.6% Leading to discontinuation: 1.7% Liver function abnormalities: 0.9% SAEs: 1.3% Deaths: 0% | GI disorders |
| Ashida, 2018 [36] | Japan | Drug- related TEAEs: • VPZ 10 mg: 10.4% • VPZ 20 mg: 10.3% Leading to discontinuation: • VPZ 10 mg: 2.5% • VPZ 20 mg: 3.9% SAEs: • VPZ 10 mg: 2.5% • VPZ 20mg: 2.0% Deaths: • VPZ 10 mg: 0% • VPZ 20 mg: 0% | Drug-related TEAEs: 11.4% Leading to discontinuation: 4.0% SAEs: 2.0% Deaths: 0% | Nasopharyngitis |
| Kawai, 2018 [41] | Japan | Drug- related TEAEs: • VPZ 10 mg: 16.3% • VPZ 20 mg: 19.3% Leading to discontinuation: • VPZ 10 mg: 7.9% • VPZ 20 mg: 7.4% SAEs: • VPZ 10 mg: 2.0% • VPZ 20 mg: 2.0% Deaths: • VPZ 10 mg: 0.5% • VPZ 20 mg: 0% | Drug-related TEAEs: 24.4% Leading to discontinuation: 9.2% SAEs: 1.4% Deaths: 0% | Nasopharyngitis |
| Mizokami, 2018 [35] | Japan | Drug- related TEAEs: • VPZ 10 mg: 17.4% • VPZ 20 mg: 17.5% Leading to discontinuation: • VPZ 10 mg: 4.1% • VPZ 20 mg: 12.7% SAEs: • VPZ 10 mg: 0.9% • VPZ 20 mg: 0.9% | Drug-related TEAEs: 19.0% Leading to discontinuation: 7.6% SAEs: 0% | Nasopharyngitis |
| Ashida, 2016 [38] | Japan | Drug- related TEAEs: 6.8% Leading to discontinuation: 1.0% SAEs: 0% Death: 0% | Drug-related TEAEs: 5.9% Leading to discontinuation: 1.5% SAEs: 1.5% Death: 0% | NA |
| Ashida, 2015 [39] | Japan | Drug- related TEAEs: • VPZ 5 mg: 6.1% • VPZ 10 mg: 9.0% • VPZ 20 mg: 10.4% • VPZ 40 mg: 4.8% Leading to discontinuation: • VPZ 5 mg: 0.7% • VPZ 10 mg: 3.4% • VPZ 20 mg: 7.1% • VPZ 40 mg: 1.4% SAEs: • VPZ 5 mg: 0.7% • VPZ 10 mg: 0% • VPZ 20 mg: 1.9% • VPZ 40 mg: 1.4% | Drug- related TEAEs: 5.8% Leading to discontinuation: 2.9% SAEs: 0.7% | Nasopharyngitis |
Conclusions
Vonoprazan is a new drug of the P-CABs class approved for the management of acid-related diseases in Brazil. Considering the difficulties encountered in attaining effective symptomatic control, such as night-time reflux, acidic residual reflux, and others, using currently available PPIs, this new class of drugs, which achieves rapid, potent, and prolonged acid suppression (including in the night-time), suggests meeting some unmet clinical needs in GERD treatment. According to the current reviewed material, vonoprazan is a valuable tool for managing these conditions while maintaining a comparably known safety profile to PPIs.









