INTRODUCTION
Severe asthma is characterized by poor symptom control and frequent recurrent exacerbations despite high-dose inhaled corticosteroids (ICS) and a second controller agent. Severe asthma prevalence is less than 5% of all asthmatics, but causes frequent hospitalizations, frequent emergency department visits, and frequent use of systemic corticosteroids due to exacerbations [1–3]. According to the national database records in Türkiye, there is a higher rate of asthma patients with severe asthma (38%) [4]. Omalizumab, mepolizumab and benralizumab are biological agents approved for the treatment of severe asthma in Türkiye. With the increasing use of biological agents in the treatment of severe asthma, reductions in asthma exacerbation rates, improvement in symptom control, improvement in quality of life, and improvements in pulmonary function tests have been observed, and this has been proven by real-life studies and randomized clinical trials [5–9].
Primary immunodeficiency with antibody deficiencies are the most common immunodeficiency phenotypes in adult patients and are characterized by frequent recurrent lung infections [10]. Asthma is a common comorbid disease in primary immunodeficiency with antibody deficiencies [11]. In a recent study conducted in Türkiye, the prevalence of asthma was found to be 22%, and the most common lung involvement in adult patients with primary immunodeficiency was observed to be bronchiectasis [12]. Asthmatic patients with antibody deficiency are at higher risk of asthma exacerbations and are more likely to have severe asthma [13]. Prophylactic antibiotic therapy or intravenous immunoglobulin (IVIG) replacement therapy is the standard treatment for patients with primary immunodeficiency who have antibody deficiency [13, 14]. In our country, awareness of immunodeficiency is low among physicians [15].
The case is presented here of a patient with severe eosinophilic asthma and concomitant immunoglobulin subclass deficiency who was successfully treated with benralizumab and IVIG.
CASE REPORT
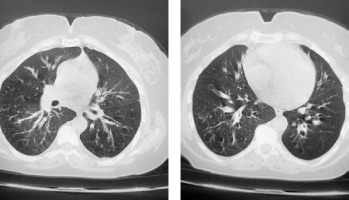
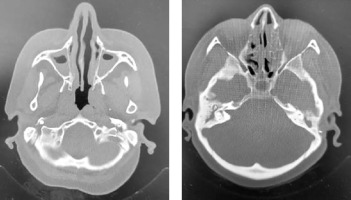
A 47-year-old female patient presented at our adult allergy and immunology outpatient clinic with complaints of cough, sputum production, and shortness of breath. Her medical history included asthma and allergic rhinitis for 15 years. The patient was using salmeterol fluticasone 500/50 µg 2 × 1, desloratadine 5 mg 1 × 1, montelukast 10 mg 1 × 1, and salbutamol inhaler 100 µg 4 × 1 for the diagnosis of asthma and allergic rhinitis. The patient has had two asthma exacerbations requiring oral corticosteroids (OCS) (at least 3 days or longer) in the last year. There was also a history of frequent antibiotic use due to frequently recurring pulmonary infections. Rhonchi were detected on both lungs during the physical examination. At the time of presentation at the clinic, the patient’s asthma was not under control, with an asthma control test (ACT) score of 5. In laboratory tests, total IgE, blood eosinophil count and house dust mite specific IgE level was high, and IgG2 and IgG4 levels were low. In flow cytometric analysis, CD3, CD4, CD8, CD16/56, CD19 values were within normal range. Both PR3 anti-neutrophil cytoplasmic antibody (ANCA) and MPO ANCA were detected negative. HIV serology was detected negative. Complete urinalysis examination and other laboratory tests were within normal range. Bronchiectasis in both lungs was determined on thoracic computed tomography (Figure 1). On paranasal sinus computed tomography, findings were consistent with widespread sinusitis in all paranasal sinuses (Figure 2). In the pulmonary function test, forced expiratory volume in 1 s (FEV1) was 34%, forced vital capacity (FVC) was 53%, and the FEV1/FVC ratio was 55%. The patient was diagnosed with uncontrolled hypereosinophilic severe persistent asthma and immune deficiency (immunoglobulin G subclass deficiency). The patient had frequent lung infections under antibiotic prophylaxis with trimethoprim-sulfamethoxazole 80/400 mg 3 days per week. Treatment was started of benralizumab 30 mg/subcutaneously once a month for the diagnosis of uncontrolled hypereosinophilic severe persistent asthma, and IVIG 30 g every 21 days for the diagnosis of immunodeficiency (ACT score at 1 year: 20). After 1 year of benralizumab treatment, improvements were observed in respiratory function tests (FEV1: 46%, FVC: 65% and FEV1/FVC ratio: 64%) and blood eosinophil count (at 12th month: 0 cell/ml). The IVIG treatment resulted in decreased antibiotic use for recurrent lung infections in the 1-year period. The clinical and laboratory findings of the patient are shown in Table 1.
Table 1
General characteristics of the patient
[i] BMI – body mass index, ICS – inhaled corticosteroids, LABA – long acting β agonist, SABA – short acting β agonist, AH – antihistamines, LTRA – leukotriene receptor antagonists, OCS – oral corticosteroids, FEV1 – forced expiratory volume in 1 s, FVC – forced vital capacity, ACT – Asthma Control Test, ANCA – anti-neutrophil cytoplasmic antibody.
DISCUSSION
This case report describes a patient with severe eosinophilic asthma and accompanying immune deficiency (immunoglobulin G subclass deficiency), for whom asthma control was achieved with benralizumab and the frequency of recurrent pulmonary infections was reduced with intravenous immunoglobulin. To the best of our knowledge, this is the first case in the literature from Türkiye in which a patient with severe asthma and concomitant immunoglobulin G subclass deficiency achieved successful asthma control with benralizumab. There are very few studies in the literature about cases diagnosed with severe asthma and accompanying primary immunodeficiency who have been treated with biological agents for asthma.
Adatia et al. initiated benralizumab treatment in a 58-year-old patient diagnosed with autosomal dominant STAT3 mutation-positive hyper IgE syndrome and uncontrolled eosinophilic asthma. After 3 months of treatment, a decrease in sputum eosinophilia was observed, cough and sputum complaints decreased, and so did the need for systemic steroids, which had previously been used regularly [16].
Lan et al. started omalizumab treatment at a dose of 300 mg every 2 weeks for a 28-year-old female with a 7-year history of chronic cough, recurrent pulmonary infections, dermatitis, skin abscesses, asthma, allergic bronchopulmonary aspergillosis, a high serum total IgE level and a diagnosis of autosomal dominant hyper IgE syndrome with positive STAT3 mutation. During follow-up, the patient showed improvements in respiratory symptoms and pulmonary function tests, the serum total IgE level remained stable, and decreases were observed in radiological infiltrates [17].
Tiotiu et al. evaluated the efficacy of biological agents in severe asthmatic patients with and without antibody deficiencies. After 6 months of treatment with biological agents, a decrease in asthma exacerbations, emergency department admissions and hospitalizations due to asthma exacerbations, and use of systemic steroids because of asthma, together with improvements in asthma control questionnaire scores, and increases in FEV1 were seen. These improvements were seen to be less in severe asthmatic patients with antibody deficiencies than in severe asthmatic patients without antibody deficiencies. It was therefore emphasized that severe asthmatic patients with antibody deficiencies may be less responsive to biological treatments [18].
Gomes et al. started omalizumab treatment every 2 weeks for 2 patients: a 33-year-old patient diagnosed with STAT3 mutation-positive hyper IgE syndrome and a 39-year-old patient diagnosed with DOCK8 gene mutation. In both patients, a decrease was observed in skin lesions and pruritus, and respiratory symptoms [19].
Jesenak et al. treated a 15-year-old girl with DiGeorge syndrome and severe allergic asthma with omalizumab at a dose of 300 mg every 4 weeks in addition to standard asthma treatment. A decrease in the number of respiratory tract infections, an improvement in quality of life, a decrease in asthma exacerbations, and improvements in pulmonary function tests after omalizumab treatment were observed, with no side-effects reported [20].
A 41-year-old patient with severe asthma and a history of atopic dermatitis and secondary immunodeficiency was started on dupilumab treatment by Brodska et al. A decrease was observed in the total IgE level, an increase in asthma control test scores and FEV1, and improvements in quality of life, pruritus, sleep quality, anxiety and depression [21].
Previous studies of patients with concomitant immunodeficiency who were treated with biological agents for severe asthma are summarized in Table 2.
Table 2
Previous studies of patients with concomitant immunodeficiency who were treated with biological agents for severe asthma
| References | Study | Disease | Biological Agent | Results |
|---|---|---|---|---|
| Adatia et al. [16] | Case report | Hyper IgE syndrome Severe asthma | Benralizumab |
Complaints |
| Lan et al. [17] | Case report | Hyper IgE syndrome Severe asthma Allergic bronchopulmonary aspergillosis | Omalizumab | |
| Tiotiu et al. [18] | Original article | Humoral immunodeficiencies Severe asthma | Omalizumab Mepolizumab Benralizumab Dupilumab Tezepelumab | |
| Gomes et al. [19] | Case report | Hyper IgE syndrome Severe asthma | Omalizumab | |
| Jesenak et al. [20] | Case report | DiGeorge syndrome Severe asthma | Omalizumab | |
| Brodska et al. [21] | Case report | Atopic dermatitis Secondary immunodeficiency Severe asthma | Dupilumab |
CONCLUSIONS
Severe asthma may be accompanied by immunodeficiency, and severe asthma may be seen in immunocompromised patients. If there is an accompanying immunodeficiency in severe asthmatic patients, frequent lung infections may affect asthma control, and similarly, uncontrolled asthma symptoms in immunocompromised patients may increase the tendency to infections. Therefore, in cases of severe asthma and accompanying immunodeficiency, asthma treatment and immunodeficiency treatment should be performed together, and biological agent treatments may be required for asthma control.