Introduction
About 85% to 90% of the cancers related to the head of the pancreas, distal bile duct, and distal stomach or duodenum or papilla of Vater are unresectable at the time of diagnosis [1, 2]. Most of such patients have locally advanced or metastatic cancer with a median survival of 3 to 6 months only [3, 4]. They often have a low quality of life (QOL) because of gastric outlet obstruction (GOO), pain, and jaundice [5]. Hence, ameliorating symptoms is the primary aim in these patients.
GOO is one of the common symptoms/complications of these tumours. Surgical intervention is associated with significant morbidity and mortality rates in these patients due to advanced disease and low performance scores (PS) [6, 7]. Post-gastrojejunostomy delayed gastric emptying is seen in about 57% of patients [8, 9]. Hence, alternative modalities of palliation have been adopted.
Endoscopic placement of a self-expandable metal stent has emerged as one of the best alternative treatment options in cases of upper intestinal obstruction [10, 11] due to high technical and clinical success rates of 94% to 97% and 87% to 94%, respectively [12, 13]. Also, it is associated with short hospital stay and almost zero intervention-related deaths. Oral intake can be started within 24 h of stent placement. The success and acceptance rates have improved further with newer stents like the Wallflex from Boston Scientific.
Our hospital, being the largest tertiary care cancer hospital in India, gets a lot of referrals of GOO patients from the whole country. Hence, we place lot of such stents in our patients for nutrition and palliation of symptoms. Approximately 5 to 7 such stents are placed every month. However, not much literature is available about them in India. Hence, we took up this study to see the technical and clinical success in our patients with malignant GOO using Boston Scientific’s Walflex Duodenal stents.
This is perhaps the first such large study on the enteral WallFlex stent in India since its inception in 2010.
Aim
We took up this study to find the technical and clinical success, and complication rates of duodenal stenting in such patients presented at India’s largest tertiary care cancer hospital.
Material and methods
Patients
This retrospective observational study included all patients who underwent endoscopic placement of an enteral WallFlex stent for malignant GOO between April 2013 and February 2019 at Tata Memorial Cancer Hospital, Mumbai, India. Inclusion criteria were as follows: (i) unresectable or metastatic malignancy with GOO (GOO score 0, 1, or 2 (Table I) [14]; (ii) not fit or willing to undergo surgical gastrojejunostomy. Exclusion criteria were as follows: (i) previous gastric or duodenal surgery other than Billroth-I reconstruction; (ii) evidence of other strictures in the gastrointestinal tract; (iii) previous gastrojejunostomy or stent placement as palliative treatment for the same condition; and (iv) clinically significant bleeding from the malignancy; (v) oesophageal or GE junction stricture.
Table I
Gastric outlet obstruction scoring system (GOOSS)
| 0 | No oral intake |
| 1 | Liquids only |
| 2 | Soft solids |
| 3 | Low-residue or full diet |
Written informed consent was obtained from all patients before the procedure. The Institutional Ethics Committee approved this study.
Definitions of GOO score
For estimation of the ability for oral intake, a GOO scoring system (GOOS) was used [14] (Table I). Technical success was defined as correct placement of the fully opened stent across the stricture, confirmed after 24 h on X-ray. Clinical success was defined as an increase in the GOO score to 2 or 3. Recurrence of obstructive symptoms was defined as a decrease in the GOO score to less than 2.
Instruments and procedures
Therapeutic duodenoscope (TJF-150 Olympus, Tokyo, Japan) or paediatric colonoscope (CF Q 160 Olympus, Tokyo, Japan) was used for WallFlex stent placement. All the procedures were done by 2 expert consultants, Dr. Shaesta Mehta and Dr. Prachi Patil, with more than 11 years of experience in our department and assisted by the senior residents. All procedures were done under anaesthetist-guided sedation.
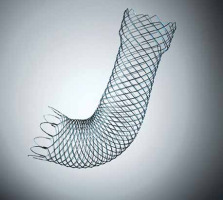
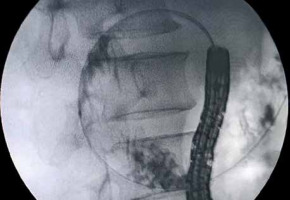
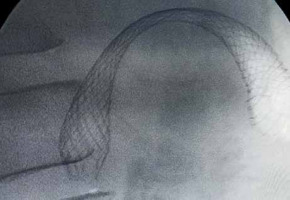
WallFlex is a braided, uncovered, self-expandable metal stent made of nitinol, with a diameter of 22 mm and lengths of 60, 90, or 120 mm. Its delivery catheter is 3.3 mm in diameter, permitting stent placement through the scope’s working channel (Figure 1). All patients included in this study received stent placement in a through-the-scope manner. A guidewire was passed through the stricture with a standard endoscopic retrograde cholangiopancreatography (ERCP). After the guidewire had been inserted deeply into the jejunum under fluoroscopic guidance, water-soluble radiographic contrast was injected through the catheter to define the stricture characteristics (Figure 2). Subsequently, the stent was deployed over the guidewire (Figure 3).
Data collection
Data were retrospectively obtained from hospital records, endoscopy/fluoroscopy reports, and follow-up clinical visits after stent placement. Technical success rate, procedure-related complications, clinical success rate, time to recurrence of obstructive symptoms, and patient survival were estimated.
Results
Initially, 269 cases were screened for the study. Of these, 234 patients were studied further after applying exclusion criteria. Twenty patients were excluded later due to lack of complete data. Patient characteristics are listed in Table II. In all patients, the clinical stage of the primary disease was Stage IV or locally inoperable cancer with GOO.
Table II
Baseline characteristics and demographics of the patients in the study
The mean age was 62.5 years, with a range of 32 to 81 years. The diagnoses were inoperable malignancies in all cases and included pancreatic/periampullary/ampullary 113 (52.80%), gall bladder 51 (23.83%), gastric 30 (14.01%), duodenal 12(5.60%), and other malignancies like GIST, leiomyomas, etc. in 8 (3.73%). The sites of malignant obstruction were as follows: D1-D2 junction/D2 in 104 (48.59%), the first part of D1 in 65 (30.37%), pylorus in 26 (12.14%), and the third part of the duodenum in 16 (7.47%) patients.
Technical success and complications
The technical success rate was 98.13% (210/214). 90-mm and 120-mm stents were placed in 167 (78.03%) and 47 (21.96%) patients, respectively. The mean procedure time was 30.6 min (standard deviation (SD):, 10.8 min). Procedure-related bleeding occurred in 12 (5.60%) of the patients, which was mild and stopped on its own or after injection therapy. Four (1.86%) patients with gallbladder cancer developed mild post-procedural pancreatitis. Eight (3.80%) of the patients developed sedation-related complications like hypotension, desaturation, and aspiration pneumonia, etc., which were treated conservatively. None required ICU admission. No perforation occurred. In 10 (4.67%) patients, stent migrations were observed during a mean follow-up period of 120 days (Table III).
Table III
Results of previous studies
| Author | Year | N | Technical success (%) | Complications (%) | Clinical success rate (%) | Time to dysfunction |
|---|---|---|---|---|---|---|
| Piesman et al. [19] | 2009 | 43 | 100 | 9 | 80 | |
| van Hooft et al. [20] | 2009 | 51 | 98 | 12 | 84 | 307 days |
| Jeurnink et al. [21] | 2010 | 21 | 95 | 5 | 62 | 50 days |
| Moura et al. [22] | 2012 | 15 | 5 | 2.4 months (mean) | ||
| Kanno et al. [14] | 2012 | 21 | 100 | 81 | 148 days | |
| Present study | 2019 | 214 | 98.13 | 11.05 | 91.42 | 130 |
In 42 patients, nasogastric tube drainage was required before the intervention. The clinical success rate, defined as well tolerating prosthetic diet, was about 91.42% (192/210). In the clinically successful group, the median time to an increased GOO score of 2 after the procedure was 5 days (range: 2–14 days). In the mean follow-up period of 120 days (range from 90 to 270 days), recurrence of obstructive symptoms was observed in 66 (31.42%) of the patients. The median time between clinical success and recurrence of obstructive symptoms was 130 days (95% confidence interval (CI): 14–210) by the Kaplan-Meier method. All patients who reported recurrence of symptoms underwent EGD and X-ray or CECT of the abdomen. There was tumour ingrowth through the meshwork of the stent in 59.09% (39/66), food impaction in 31.81% (21/66), and migration of the stents in 15.15% (10/66) of the cases as reasons for recurrence of symptoms. The median patient survival was 98 days (95% CI: 60–360).
Discussion
Malignant GOO occurs in the last stages of various malignancies. GOO induces intractable nausea and vomiting, abdominal distension, and decreased oral intake, resulting in a lower QOL. Nasogastric tube drainage lessens vomiting and abdominal distension but does not resolve malnutrition and electrolyte imbalance. Surgical gastrojejunostomy has been commonly used as a palliative treatment for malignant GOO. Recently, endoscopic placement of an enteral self-expandable metal stent has been reported as a feasible option for GOO palliation [14]. There have been 4 randomized controlled trials comparing surgical gastrojejunostomy with endoscopic stent placement [15–17]. Most of them concluded that endoscopic stent placement was preferable to gastrojejunostomy for a more rapid recovery of oral intake but unfavourable due to the duration of sustaining sufficient oral intake. Therefore, gastrojejunostomy is suitable for patients with a long life expectancy, whereas endoscopic stent placement is preferable for those with a short life expectancy. For those who seem intolerant to surgical gastrojejunostomy, endoscopic stent placement is the only possible palliation to achieve oral feeding recovery.
In our study, pancreatic and periampullary malignancies were the most typical causes of malignant GOO, which required enteral stenting. This corresponds to the high prevalence of these malignancies in our region and the fact that they present late to us. The most common obstruction sites were at the D1-D2 junction and the 2nd part of the duodenum (D2), followed by D1, pylorus, and D3. Similar results were seen in a study by Yim, in which pancreatic malignancies were the main indication for enteral stenting, and duodenum (D1/D2) was the main site of obstruction [18]. However, we did not find any Mets causing GOO in our study, unlike in the above study where Mets caused GOO in 27.6% of patients.
The technical success in our study was 98.13% (210/214). In 3 patients, we failed because even a guidewire could not be passed across the stricture. In 1 patient, the stent could not open after deployment. 90-mm and 120-mm stents were placed in 167 (78.03%) and 47 (21.96%) patients, respectively. Two patients required 2 stents because the 90-mm stent first deployed had not covered the stenosis’s oral side due to stent shortening. Hence, a 120-mm stent was placed within this stent to cover that. The mean procedure time was 30.6 min (SD = 10.8 min). This is slightly more than in the study by Kanno et al. [14], which reported only 20 min. This may be due to more experience and technical know-how in their centre. We also noted a duration of procedures performed in later years.
Reported complications of enteral stent insertion have been minimal in nature. Examples include ulceration caused by the stent wires, bleeding, migration, occlusion, and perforation [18]. In our study, procedure-related bleeding occured in 12 (5.60%) of the patients, which was mild and stopped on its own or after injection therapy. Four (1.86%) patients with gallbladder cancer developed mild post-procedural pancreatitis. In 10 (4.67%) patients, stent migrations were observed during a mean follow-up period of 120 days. Thirty-five (16.66%) patients felt mild to moderate pain in the abdomen after stent deployment, which lasted for about 3.5 days on an average (range: 1 to 9 days). All patients responded to standard dosages of analgesics. These results are comparable with other studies [14, 18].
Furthermore, 8 (3.80%) of our patients developed sedation-related complications like hypotension, desaturation, aspiration pneumonia, etc., which were treated conservatively. We could not find any study that looked at or mentioned this complication during enteral stenting.
The clinical success rate, defined as a tolerating prosthetic diet, was about 91.42% (192/210), which is comparable with other studies [14, 18]. After enteral stent placement, the mean GOOS score significantly improved compared with pre-treatment scores. The median time to an increased GOO score of 2 after the procedure was 5 days (range: 2–14 days). This may be because the stent fully opens in about 2 to 5 days. In the mean follow-up period of 120 days (range: 90 to 270 days), recurrence of obstructive symptoms was observed in 32 (31.68%) of the patients. The median time between clinical success and recurrence of obstructive symptoms was 130 days (95% CI: 14–210) by the Kaplan-Meier method. However, in a study by Kanno, in a mean follow-up period of 101 days (SD = 125 days), recurrence of obstructive symptoms was observed in 47% of the patients [8, 17]. The median time between clinical success and recurrence of obstructive symptoms was 148 days (95% CI: 0–328) by the Kaplan-Meier method. All patients who reported recurrence of symptoms underwent EGD and X-ray or CECT of the abdomen. There was tumour ingrowth through the meshwork of the stent in 59.09% (39/66), food impaction in 31.81% (21/66), and migration of the stents in 15.15% (10/66) of the cases as reasons for recurrence of symptoms. All the patients with food impaction and stent migration underwent stent re-insertion, and all were successful. However, only 19 patients with tumour ingrowth underwent stent re-insertion for various reasons like refusal by the patient or family, financial constraints, etc., and all were successful. In total, 152 (72.38%) of the patients received chemotherapy after improving their performance score (PS) after stent deployment. The median patient survival was 98 days (95% CI: 60–360).
Limitations of this study: it was a single retrospective centre observational study. No controls were used. To confirm the superiority of stents versus surgery, a randomized prospective multicentre study would be most suitable.