INTRODUCTION
Worldwide, more than 25 million people are currently affected by dementia, most of them suffering from Alzheimer’s disease (AD). The affected population constitutes a growing global health problem with significant negative consequences for individuals and society. Despite progress in the pharmacological treatment of AD, the prognosis is generally poor. The disease inevitably leads to marked disability, dependence, and finally death. Therefore, much attention has been paid in recent decades to the development of novel medications and the introduction of other non-pharmacological interventions.
It is generally agreed that physical activity (PA) plays a role in the prevention of many medical conditions and mental diseases. It has been postulated that different forms of physical exercises may prevent, alleviate, or at least delay the progression of AD. Numerous studies assessing the relationship between PA and AD have been published in the last two decades, but their results are still inconclusive.
The effect of PA on the cognitive functioning of people with AD was the aim of the meta-analysis conducted by Heyn et al. [1]. Thirty randomized trials, which included 2020 subjects with cognitive impairment (MMSE < 25), aged 65 and over, who participated in at least 4 weeks of training were analyzed. The authors demonstrated a positive effect of exercise on cognition, exercise capacity, and behavioral disorders accompanying dementia. Another systematic review and meta-analysis of 13 (n = 869) randomized controlled trials (RCTs) have also provided evidence for a positive effect of exercise on the cognitive function of patients with AD [2]. These findings were confirmed by the authors of a more recent meta-analysis [3] which included 13 randomized controlled trials (n = 673).
Authors of another meta-analysis [4] found that in comparison to drug treatment (cholinesterase inhibitors, Memantine, and Ginkgo biloba) which had a small, pooled effect on cognition of AD patients and people with mild cognitive impairment (MCI), exercise training resulted in moderate to strong pooled effect size in AD patients.
Many risk factors and protective factors have been linked with the pathogenesis of AD. In this context, the goal of another interesting line of research is to explore whether PA can decrease the risk of AD. Results of a meta-analysis published by Santos-Lozano [5] based on 10 high-quality studies (N = 23345) suggest that despite the limitations related to the quality of the obtained data, a regular PA may play a protective role in case of the elderly. However, the role of PA should be considered in the context of other protective factors. The aim of a systematic review and meta-analysis of 243 observational prospective studies and 153 randomized controlled trials was to assess the potential role of 104 modifiable factors and 11 interventions in AD protection [6]. The authors found 19 potentially protective factors among them 10 (i.a. education, cognitive activity, high body mass index in late life, hyperhomocysteinemia, depression) were recognized as Level A (strong evidence) while physical exercise was found as Level B (weaker evidence) together with i.a.: obesity in midlife, weight loss in late life, smoking, sleep, cerebrovascular disease.
It turns out, however, that the relationship between PA and AD is probably more complex. A 28-year observational study [7], in which the physical activity of 10,308 people was assessed every four years, in parallel with the monitoring of their cognitive functions showed that nine years before the diagnosis of dementia, a gradual decline in PA began – regardless (!) of its initial level. From that perspective, a reduction in PA may be considered rather as a prodrome of dementia than a risk factor for the disease.
In contrast, other studies cast doubt on the importance of PA as a protective agent for dementia. Tan et al. [8] reviewed data on a potential relationship between PA and risk of dementia collected in the Framingham study. During a 10-year follow-up of 2,063 subjects, the authors found that even mild PA was associated with a lower risk of being diagnosed with dementia, but this was not the case for people with the APOE-ε4 genotype. These data imply a linear relationship between PA in old age and the volume of the hippocampus. However, extending the follow-up to 22 years reduced the relationship between PA and the risk of AD. The results of other meta-analyses [9-11] also question the importance of the protective effect of PA. A meta- analysis by Frederiksen et al. [12] showed that there is no evidence that PA affects the volume of the hippocampus, Aβ accumulation, and the concentration of tau protein in the cerebrospinal fluid. A one-year controlled study by Lamb et al. [13] included 494 subjects (65% men) with mild to moderate dementia. Some of them (n = 329) were assigned to the group taking moderate or high-level PA while the other participants were provided with standard care. In the active group, a slight but statistically significant faster progression of the disease was demonstrated. Moreover, no improvement in the results obtained with the Alzheimer’s Disease Cooperative Study – Activity of Daily Living scale (ADL) was found.
Many methodological problems of studies focused on the impact of PA on AD patients were raised by their authors, including heterogeneity of the types of physical activity or exercise, the methods and duration of interventions, its intensity, and methods of assessment of cognition. Small sample sizes and lack of RCT were also considered in this context.
The following hypotheses were adopted:
METHODS
The study was a quasi-experiment: it was the patients and their carers who decided to participate actively or passively in the study. After getting acquainted with its course, the patient and the carer who agreed to participate signed a written consent. The consent for the study was approved by the Bioethics Committee of the Medical University of Karol Marcinkowski in Poznań (decision no. 45/15 of 8 January 2015).
Outpatients from the Polish population who met the diagnostic criteria for probable AD according to ICD-10 (International Statistical Classification of Diseases, version 10) with mild to moderate severity (MMSE 11-23) were recruited for the study. The drugs used to treat the patients (Donepezil, Rivastigmine, Memantine) were taken by them in stable doses for at least 3 months prior to the beginning of the study. Another inclusion criterion was a declaration of help in conducting the study by the patient’s caregiver. Among the exclusion criteria were depression, significant somatic contraindications to PA, and a physically active lifestyle before the study. Of the 36 people who agreed to participate in the study, 31 passed all the protocol-defined testing procedures (one in 32 dropped out before the follow-up testing). 27 study participants suffered from late-onset AD (LOAD), 5 from early-onset AD (EOAD). This was the basis for distinguishing subgroups in the study, along with the participant’s gender and the stage of the disease. Finally, 16 out of 19 people (including 3 men) who initially declared active participation in the study were qualified to the active group. The training intensity in the first month was meant to be just a warm-up for the next 2 months of regular, moderate PA. Nordic walking was the recommended form of activity. It is performed with purpose-designed walking poles similar to ski sticks. Both Nordic walking and conventional walking are beneficial for older adults. However, compared to conventional walking, Nordic walking provides additional benefits, such as improving aerobic capacity and muscular strength as well as other components of functional fitness in a short period. Nordic walkers use more of their entire body and receive fitness building stimulation for the chest, upper extremities, abdominals, spinal, and other core muscles, which is absent from normal walking, The tutor, who was a living-in family member of the patient in most cases, supervised the duration, intensity and regularity of PA, following the guidelines received from the researchers. Based on the results of studies conducted by other researchers [14, 15], the minimum PA in the second and third month was adopted as the criterion for qualifying the patient to the active group at 30 minutes three times a week. The primary treatment for AD was not modified during the study. Daily activity and the severity of cognitive impairment were assessed at the beginning of the study and 3 months after its completion.
Psychometric measurements
When qualifying the potential candidates for the study, the previously obtained results in the Mental State Assessment Short Scale (MMSE) were used. This scale was not reused during the study.
Activities of Daily Living (ADL)
General functioning was assessed using Activities of Daily Living scale [16], which is a questionnaire that allows for a precise assessment of the degree of independence and efficiency of patients, based on information obtained from the caregiver. The assessment covers the patient’s daily activities over the past month. A non-disabled person scores 78 points. It usually takes 10 to 15 minutes to conduct the interview necessary to complete the questionnaire.
Montreal Cognitive Assessment scale (MoCA)
The severity of cognitive deficits was assessed with the use of the Montreal Cognitive Assessment scale [17, 18], which assesses the following cognitive functions: orientation, visuo-spatial abilities, executive, short-term memory, verbal fluency, attention, and abstraction. It takes 10 to 15 minutes to complete the MoCA test, which in the current study was performed twice: at the beginning and the end of the study, using two different, equivalent versions to eliminate the measurement error resulting from learning.
Statistical analysis
The Shapiro-Wilk test was used to determine whether the data could be approximated by a normal distribution, and the Bartlett test to determine the homoscedasticity of variance (equality of variance between groups). As only a small number of features showed a normal distribution, it was decided to apply the non-parametric methods of statistical inference. To compare the data at the start and end of the study in the active and passive groups, the Wilcoxon test was applied to the paired data, while the comparison of the active and passive groups was performed using the Mann-Whitney test. The test power (probability of rejecting the null hypothesis if false) calculated using GPower (version 3.1) is given in parentheses for each significance value. Correlation analysis was performed using the Spearman correlation method, while in the case of the correlation coefficient significance test, a multiple test correction was applied. In all statistical tests, the level of significance was α < 0.05.
RESULTS
Demographic and clinical characteristics of patients
As can be seen from the data in the Table 1, there are no differences between the active and passive groups in terms of clinical or demographic characteristics. The only difference was in education: in the active group, four people had higher education; there were no such people in the inactive group, while four more people had primary education in this group.
Table 1
Demographic and clinical characteristics of patients
The influence of physical activity on general functioning – ADl scale
There were no statistically significant differences between the ADL measurements at the beginning and at the end of the study, both in the entire study population and in the active and inactive groups, and in the case of the initial severity of cognitive deficits (MMSE score) as an additional criterion (Figure I). Taking into account both the activity and gender of the patients, the ADL scale showed statistically significant deterioration in all men (active and inactive) (Wilcoxon test, p = 0.035, test power 47%).
Figure I
The effect of physical activity on general functioning – ADL scale (The Alzheimer’s Disease Cooperative Study – Activities of Daily Living). ADL1 act. – score of active participants in the ADL scale before training, ADL2 act. – score of active participants in the ADL scale after 3-month of training, ADL1 cont. – score of non-active participants in the ADL scale at the beginning of the study, ADL2 cont. – score of non-active participants in the ADL scale after 3 months
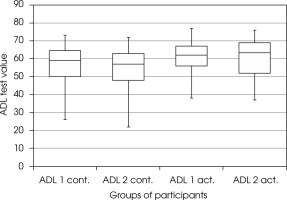
In the group of patients with the LOAD (N = 27), no statistically significant differences were found between ADL measurements at the beginning and the end of the study, also when divided into the active and inactive group. Taking into account the additional criteria for the division of this group – the activity and gender of patients – statistically significant deterioration was found in all men (active and inactive; Wilcoxon test, p = 0.035, test power 47%).
The influence of physical activity on cognitive functions – moCA scale
There were no statistically significant differences between the MoCA measurements at the beginning and the end of the study in the active and passive groups (Wilcoxon test p = 0.140 and p = 0.470, test power 27% and 16%, respectively) (Figure II). Taking into account the criteria of gender and physical activity also did not allow for finding statistically significant differences between the results obtained by the groups at the beginning and the end of the study.
Figure II
The effect of physical activity on cognitive functions – MoCA scale (Montreal Cognitive Assessment scale). MoCA1 act. – score of active participants in MoCA scale before training, MoCA2 act. – score of active participants on the MoCA scale after 3-month training. MoCA1 cont. – score of non-active participants in the MoCA scale at the beginning of the study, MoCA2 cont. – score of inactive participants in the MoCA scale after 3 months
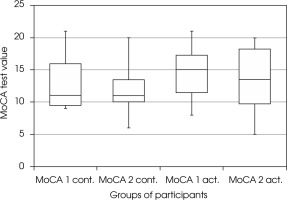
In the active group of participants with moderate dementia (MMSE 11-18), the cognitive deficits increased (Wilcoxon test p = 0.040, test power 63%).
In 27 patients with LOAD a statistically significant deterioration in the MoCA scale was found in both study subgroups (active and inactive; decrease from 14 to 13 points, p = 0.036, test power 52%). There was a statistically significant decrease from 15 to 14 points in the MoCA scale in the active group (p = 0.020, test power 75%), but not in the inactive group of women with LOAD. No statistically significant differences were found in the remaining groups.
DISCUSSION
The aim of the study was to determine whether moderate exercise in the form of Nordic walking, taken over the period 3 months, would improve the general functioning and cognitive functions of people with mild and moderate AD.
There were no statistically significant differences between the ADL measurements at the beginning and the end of the study in the entire active and inactive groups, but deterioration was found in both groups of men. The decrease in ADL scores was also found in the group of men with LOAD. These results would be consistent with the results obtained in a 3-month study of 82 patients with AD, conducted by Roach et al. [19], which consisted of regular walks for 20 minutes a day under the supervision of caregivers. Different results were obtained by Holthoff et al. [20] – no progression of ADL assessed in the ADL scale was observed in the exercising participants at weeks 12 and 24, as opposed to the control group, in which the ADL result deteriorated. Due to the progressive nature of the disease, the lack of progression in comparison to the control group should be treated as a positive effect of the undertaken activity. Similar results were obtained by Vidoni et al. [21]. During the 6-month study, the active group showed an increase of 1% in the ADL score, while the control group showed an 8% decrease. No such connection was found in the present study; perhaps a longer or more intense training would be necessary to observe a link of this kind.
Rao et al. [22] reviewed 6 studies on exercise involving the total of 446 people with mild to moderate dementia, lasting from 3 to 12 months, in which the ADL scale was the primary research tool. It was found that the disease progression was inhibited in physically active people while progress was observed in the control group. The authors rated the overall effectiveness of aerobic exercise as significant. The best results were achieved by short programs lasting 3 months, promoting more vigorous walks four times a week for 30 minutes [14, 15]. Patient cooperation was found to be good, which was influenced by the involvement of caregivers in the exercise. In the cited study, the tolerance of aerobic exercise in patients was assessed as excellent. The study was very similar to the one presented in terms of duration, severity, form of PA, and its control. The positive impact of physical activation programs continued even for two years after their completion. In the 24-week RCT [20] of people with mild or moderate AD, after 12 weeks a significant difference in ADL scores was shown between the active and passive groups – the active group maintained its performance unchanged, while the disease progressed in the inactive group.
Concerning the above, it could be expected that the time and intensity of training specified in the protocol of the presented study will allow to determine the possible impact of physical activity on the general functioning and cognitive performance of patients. As a side note, it can be observed that the improvement of the physical condition itself may contribute to a certain extent to better ADL results because several areas of activity tested with the use of this measuring tool depend indirectly on physical fitness and motor coordination. A fairly simple form of activity recommended in the presented study was to minimize the impact of the independent variable on learning other skills, which could have happened in the case of another form of activity, used in another previously cited study [23].
The results of the present study do not support the hypothesis that increasing physical activity improves the overall functioning of AD patients.
No statistically significant differences were found between the MoCA measurements at the beginning and at the end of the present study, neither was it observed in the active and inactive or gender-dependent groups. Cognitive decline was found in some active subgroups, including active women with LOAD and the entire subgroup of active participants with moderate dementia. Cognitive decline was also observed in the entire group of people with LOAD. There was no such decrease in the entire subgroup with moderate or mild dementia (regardless of activity). Lamb et al. [13] described cognitive deficits in people with AD after 4 months of PA. The deterioration of the condition of the people in the subgroup with the LOAD (they constituted the vast majority of the study participants) is quite unusual, as the LOAD dynamics are usually lower than in the EOAD. Gender-related differences in the influence of aerobic exercise on cognitive functions were described in the study by Barha et al. [24], although the results obtained were different to those demonstrated in the present study – an improvement was noted in the group of active women.
Friedman and Tappen [25] showed that even short and light training (10 weeks and 30 minutes of walking, 3 times a week) can improve verbal skills. According to one meta-analysis [1], even a four-week training session can improve the cognitive functions of people with dementia and MCI. Santoz-Lozano et al. [5] drew similar conclusions: 40-minute training at 70% of the maximum heart rate of cycling 3 times a week for 3 months improved the MMSE results in people with mild AD. Yang et al. [26] compared the results achieved by people with mild to severe AD, aged 50 to 80 years, who underwent 3-month aerobic training with the results of people who only received education about a healthy lifestyle. The group of exercisers (25 people) obtained statistically better results in the MMSE, neuropsychiatric inventory, and the scale testing the quality of life of people with AD, while the results of the non-exercising group showed a further decrease in their cognitive performance. Training with 70% of a maximum heart rate consisted of 40 minutes of riding a stationary bike 3 times a week for 3 months. The intensity of the training was comparable to that used in this study.
The limitation of the presented study was its quasi-experimental nature. The assessment of the intensity and regularity of physical activity was based on the notes of the caregivers. It is difficult to exclude that some people in the active group exercised less intensively than recommended or declared. As the participants and their guardians were most often elderly, it was not planned to equip them with devices that would objectify the degree of physical activity. Being aware of the above limitations, the independent variable was defined on a nominal scale consisting of two levels: active and inactive. In the light of recent findings, the intensity and duration of training recommended in the present study were slightly lower than those currently defined as potentially effective in slowing down the impact of the underlying disease on cognitive functions [27, 28].
The psychometric assessment was made by a person who knew the participant’s declaration as to the nature of participation in the study, and the same person classified the respondents to the active or passive group. The small sample size resulted in a lower statistical quality of the collected data. The heterogeneity of the study group in terms of somatic diseases was another limitation, which cannot be, however, eliminated in the population of patients with dementia. The study population also differed in terms of pharmacotherapy, the severity of the underlying disease, and the type of the disease (5 subjects with EOAD, 27 with LOAD). Males accounted for 6 out of 32 study participants, which also did not correspond to the gender distribution in the general population of patients.
In the light of recent reports on the influence of physical activity on the course of AD, the lack of BDNF Val-66Met genotype determination may be a significant limitation in the interpretation of the results. People with the Val/Met genotype benefit less from PA than those with the Val/Val genotype. In the analysis of 114 cases of mentally healthy men and women aged 60 years and older by Brown et al. [29], brain volumes were correlated with the levels of physical activity declared by the respondents. In Val/Val homozygotes, higher levels of physical activity were associated with greater volumes of the hippocampus and temporal lobes, while in Met allele carriers it was associated with smaller temporal lobe volumes.
Overall, regular and moderate physical activity did not improve the condition of patients with mild to moderate Alzheimer’s disease, both in terms of overall functioning and cognition. Overall functioning deteriorated in both groups of men regardless of physical activity. There were no differences between men and women and between the active and inactive groups – neither at the beginning nor at the end of the study.






