Introduction
Distal pancreatectomy is a serious surgical procedure that is associated with a low mortality rate (< 5%) but a high percentage of morbidity, with postoperative complications ranging from 22.7% to 57% in high-volume pancreatic surgical centres [1–6].
Malnutrition is a common problem in hospitalised patients. According to the literature, 30% to 50% of hospitalised patients are malnourished. Malnutrition negatively influences patients’ prognosis and quality of life. Proper assessment of nutritional status and preoperative nutritional intervention can help to decrease the risk of postoperative complications. In order to assess the nutritional status, both objective and subjective criteria are used, including various anthropometric, clinical, and biochemical parameters [7, 8].
It is known that the immunological, inflammatory, and nutritional status of patients significantly influences the postoperative outcome. In the literature there are a lot of studies regarding the significance of nutritional status in patients following gastric, bowel, and proximal pancreatic resections [9–15]. However, to our knowledge, there are not many reports presenting the impact of nutritional status on postoperative complications in patients following distal pancreatectomy.
Aim
The aim of the study was the assessment of nutritional status in patients undergoing distal pancreatectomy using selected anthropometric, clinical, and biochemical parameters and analysis of the influence of nutritional status on the incidence of postoperative complications.
Material and methods
Patients
The analysis included 50 patients undergoing distal pancreatectomy in a large gastrointestinal surgery centre. Assessment of nutritional status was performed in patients at the time of admission to hospital, prior to surgical treatment. There were 21 (42%) men and 29 (58%) women, with a mean age of 57.34 ±12.07 (30–80) years in the analysed group. Inclusion criteria comprised: pancreatic pathology (tumour) requiring surgical removal (distal pancreatectomy) and age > 18 years. The exclusion criterion comprised: incomplete demographic and clinical data. The University Ethics Committee decided that for this type of study formal consent was unnecessary. Written, informed consent was obtained from all participants.
Study design
Information regarding deterioration of nutritional status, body weight before disease and current body weight, loss of body weight and food intake since the onset of disease, comorbidities (arterial hypertension, ischaemic heart disease, diabetes mellitus), and smoking was collected. On admission, the height and weight were measured, and laboratory blood tests were performed. The selected blood count parameters (haemoglobin, total white blood cell, lymphocyte, neutrophil, monocyte, platelet counts) and biochemical parameters (serum total protein, albumin, and C-reactive protein (CRP)) were analysed. The body mass index (BMI) was calculated. The nutritional risk according to NRS 2002 (Nutritional Risk Screening 2002) by the European Society of Parenteral and Enteral Nutrition (ESPEN) was assessed [16, 17]. The immunological parameters, neutrophil/lymphocyte ratio (NLR), platelet/lymphocyte ratio (PLR), and lymphocyte/monocyte ratio (LMR), were calculated [18]. Onodera’s nutritional prognostic index (PNI) was calculated based on the serum albumin concentration and total lymphocyte count in the peripheral blood using the following formula: 10 × level of albumin (g/dl) + 0.005 × total lymphocyte count (/mm3) [19]. The patients were divided into two groups according to the absence (group 1) or presence (group 2) of postoperative complications. Clinicopathological factors and laboratory parameters were compared between the two groups.
Ethics approval and consent to participate
The University Ethics Committee decided that for this type of study formal consent was unnecessary. Written, informed consent was obtained from all participants.
Statistical analysis
Comparison between groups was performed using Student’s t-test, the χ2 test, and the Mann-Whitney U test. The Shapiro-Wilk test was used to check for normality of the distribution. A p-value of < 0.05 was considered statistically significant. The statistical analyses were performed using Statistica® software, version 13.0 (StatSoft).
Results
The general clinical characteristics of 50 patients are presented in Table I. In the analysed group, most frequent pathology (tumour) was located within the pancreatic tail (23; 46%). Neuroendocrine tumours were the most frequent (19; 24.34%) histopathological types. In most (37 cases; 74%), resection of the pancreatic tail and trunk was performed. Distal pancreatectomy involving splenectomy was performed in 40 (80%) patients. The values of laboratory tests are presented in Table II. The values of PNI ranged from 33 to 67.5 with a mean value 51.34.
Table I
The patients’ clinicopathological characteristics
Table II
Laboratory results, nutritional and immunological parameters
Postoperative complications were noted in 15 (30%) patients. They are presented in Table III. Postoperative pancreatic fistula (POPF) was the most frequent complication (11; 22%). The pancreatic fistula of type B was the most frequently recorded in the analysed group; it was observed in 6 patients (12% of all complications and 54.54% of all pancreatic fistulas). Clinically relevant POPF (type B and C) was noted in seven (14%) patients. The POPF was defined and classified according to International Study Group on Pancreatic Fistula [20]. There were three relaparotomies in the analysed group. The first one was performed on the 17th postoperative day due to a retroperitoneal abscess. Drainage of the abscess was made. The second one was performed on the fourth postoperative day due to intraperitoneal bleeding caused by anticoagulant drugs administrated as treatment for a pulmonary embolism. Packing was performed. The mortality rate was 0%. There were three rehospitalisations (6%) due to intraabdominal fluid collections that were treated conservatively.
Table III
Postoperative complications
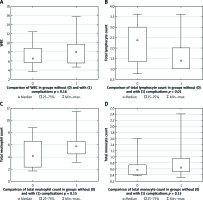
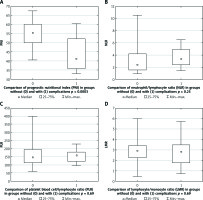
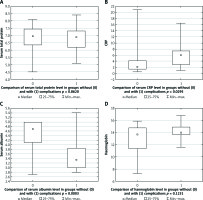
The analysed patients were divided into groups according to the presence or absence of early postoperative complications (group 1 – without complications, group 2 – with complications). The nominal and continuous variables were compared. Results of the comparison are presented in Table IV and in Figure 1–4. Both groups were comparable according to age and gender distribution. The groups were comparable according to tumour location, splenectomy, and comorbidities. Both groups were comparable according to ASA classifications (p = 0.09). Although higher ASA classifications were more frequently noted in patients with complications compared to patients without complications. The median value of the intraoperative blood loss was higher in group 2, at 650 ml, than in group 1, at 400 ml, but the difference was not statistically significant (p = 0.15). The transfusion rate was comparable in both groups (p = 0.6). The significant differences in total and postoperative hospitalisation durations between both groups were noted. The median total hospitalisation duration was 10 days in group 1 versus 18.5 days in group 2 (p = 0.0018). The median postoperative hospitalisation duration was seven days in group 1 versus 16 days in group 2 (p = 0.0008). The significant difference (p = 0.005) in NRS 2002 between both groups was noted. The higher NRS 2002 was noted in patients with postoperative complications (group 2) compared to patients without complications (group 1). Body mass index was comparable in both groups (p = 0.19). The median BMI was higher in group 2 (28 kg/m2) compared to group 1 (25 kg/m2). It should be noted that obesity was associated with higher risk of postoperative complications, but it was only a tendency, because the difference between both groups was not statistically significant. The total lymphocyte count was significantly higher in group 1 compared to group 2 (p = 0.001). The serum level of albumin was significantly higher in group 1 compared to group 2 (p = 0.0003). Also, the PNI was significantly higher in group 1 compared to group 2 (p = 0.0003). There was no significant difference in laboratory immunological parameters (NLR, LPR, LMR) in both groups. The level of C-reactive protein (CRP) was significantly higher in patients with complications compared to patients without complications (median value: 2 vs. 6 mg/dl) (p = 0.03).
Table IV
Comparison of groups without and with postoperative complications according to selected clinicopathological factors
Figure 1
Comparison of BMI (A), ASA classification (B), total hospitalisation duration (C) and postoperative hospitalisation duration (D) between patients without complications (group 1) and with complications (group 2). Mann-Whitney U test
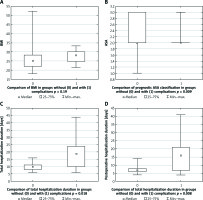
Figure 2
Comparison of serum total protein (A), C-reactive protein (CRP) (B), albumin (C), and haemoglobin (D) level between patients without complications (group 1) and with complications (group 2). Mann-Whitney U test
Discussion
In this study, we noted postoperative complications in 30% of patients. Pancreatic fistula was the most frequent complication in the analysed group. These reports are comparable with the literature data [20–23].
It is known that malnutrition is associated with disturbances within humoral and cellular immune response, and it negatively influences healing of the postoperative wound. Additionally, malnutrition is associated with poor prognosis in patients due to a higher number of postoperative complications. Therefore, it is very important to assess the nutritional status before surgical treatment. The proper assessment of nutritional status allows the selection of malnourished patients and hence preoperative nutritional intervention in order to improve nutritional status and to decrease the risk of postoperative complications [10–15, 24].
In our study, blood test parameters including total protein, albumin, haemoglobin serum levels, and lymphocyte in the peripheral blood were analysed. Hypoalbuminaemia is associated with a higher number of postoperative complications following pancreatic resection due to impaired healing and activation of inflammatory response. The lymphocyte count in the peripheral blood is the second important laboratory parameter reflecting the immunological status and inflammatory response [15, 24–28].
Hendifar et al. [28] reported that higher preoperative albumin serum concentration was associated with greater overall survival in patients with resected pancreatic cancer. There was an association between lower level of albumin and transfusion rate and duration of hospitalisation [28]. In our study, an association between lower albumin serum level and greater incidence of postoperative complications was noted. The median serum albumin level was significantly lower in patients with postoperative complications compared to patients without complications. Additionally, a significantly lower total lymphocyte count was noted in patients with complications compared to patients without complications. The values of serum albumin and total lymphocyte count were used in order to calculate the PNI.
The PNI was described in 1980 by Buzby et al. [29] (their study included patients undergoing elective surgery) in order to assess immunological and nutritional aspects of patients undergoing surgery of the digestive tract [13, 24, 29]. In 1984, Onodera et al. proposed a modified PNI calculated on the basis of two factors: serum albumin and lymphocytes in the peripheral blood [19, 24]. The authors observed that the incidence of postoperative complications was significantly higher in patients with low PNI. In 2014, Sun et al. [30] published a comprehensive meta-analysis regarding PNI. The article presented an analysis of 14 publications involving 3414 patients. The authors observed a significantly shorter overall survival and a higher number of postoperative complications in patients with low PNI but did not report a similar dependence for cancer-specific survival [28, 30]. An association between lower PNI and higher number of postoperative complications was reported in our study, i.e. there was a significant difference in PNI values between patients without and with postoperative complications.
There are a lot of publications regarding the impact of prognostic nutritional index on postoperative outcome in patients following gastrointestinal surgery including oesophageal, gastric, colorectal, and pancreatic resections [9–15, 18, 19, 25–35].
Kanda et al. [34] analysed 268 patients undergoing pancreatectomy for pancreatic cancer. In multivariable analysis, low preoperative PNI (but not low albumin) was an independent prognostic factor for poor survival. Low preoperative albumin concentration and PNI were significantly associated with postoperative complications. In this study, low PNI and low body mass index were associated with greater incidence of postoperative pancreatic fistula [34].
Sato et al. [35] analysed 44 patients undergoing distal pancreatectomy. The authors noted that rapid postoperative PNI reduction was associated with a greater incidence of postoperative pancreatic fistula. In this study, pancreatic fistula was noted in 23 (52%) patients, of which 13 (56%) were grade B or C [34].
Watanabe et al. [18] analysed inflammatory and nutritional parameters using the modified Glasgow Prognostic Score (mGPS), PNI, NLR, and PLR in 46 patients after pancreaticoduodenectomy for pancreatic cancer. In patients with PNI < 40, significantly lower haemoglobin levels and a higher number of intra-abdominal bleedings were noted. The postoperative pneumonia was more frequent in the mGPS 2 patients, and surgical complications greater than grade 3 (according to Clavien-Dindo classification) were significantly more frequent in the NLR ≥ 2.5 patients. In multivariate analysis, only the PLR was an independent prognostic indicator. There are some other publications in the literature regarding the influence of immunological status on the incidence of postoperative complications [18].
An association between higher Nutritional Risk Screening NRS 2002 and greater number of postoperative complications was noted in this study. This study also revealed higher NLR and PLR in the complications group compared to the no complications group, but these differences were not statistically significant. Regarding inflammatory status, significantly higher CRP and lower LMR (no statistical difference) were noted in patients with complications compared to patients without complications, but these differences were not statistically significant.
In our study, the median BMI was higher in patients with complications (28 kg/m2) compared to group without complications (25 kg/m2). It should be noted that overweight or obesity were associated with higher risk of postoperative complications in our study, but it was only a tendency because the difference between both groups was not statistically significant. A review of the literature data shows that the influence of BMI on the incidence of postoperative complications in patients undergoing pancreatectomy is very interesting. In some studies (e.g. Kanda et al. [34]) lower BMI was independently associated with a higher risk of postoperative pancreatic fistula. In another study, high BMI was associated with a higher risk of postoperative complications, commonly pancreatic fistula. An association of low BMI with POPF may be caused by immunological dysfunction and impaired inflammatory response, and disturbances of the wound healing in underweight patients. Vanbrugghe et al. [36] analysed 208 patients following distal pancreatectomy. The authors reported that visceral obesity with BMI ≥ 25 kg/m2 was a significant risk factor for POPF. In this study, sarcopaenia did not influence the risk of clinically relevant pancreatic fistula. An association of high BMI with POPF may be caused by difficult intraoperative conditions and soft fat pancreas in overweight or obese patients. It has been proven that soft pancreas is a risk factor for POPF. Also, overweight and obesity are associated with a higher risk of infection complications. Generally obesity negatively influences major gastroenterological surgical procedures. It has been noted that obesity is associated with prolonged operative time and might be a risk factor for short-term complications; however, an adverse influence of obesity on long-term surgical outcomes was not observed [36–38].
There are also other studies regarding the influence of nutritional status on short-term outcome after pancreatectomy. The study by Kato et al. [39] conducted on 344 patients undergoing pancreatectomy for pancreatic cancer did not show an influence of nutritional status on postoperative complications. The correlations between the Controlling Nutritional Status score (CONUT) and postoperative complications were analysed. The authors concluded that nutritional status assessed as CONUT was associated with survival in patients with pancreatic cancer after pancreatectomy but was not associated with recurrence or postoperative complications [39]. The CONUT score is a scoring nutritional system based on calculation from the following three parameters: serum albumin level, total peripheral lymphocyte count, and total cholesterol level. It is categorised into normal nutrition status (0–1 score), mild nutrition status (2–4 score), moderate nutrition status (5–8 score), and severe nutrition status (more than 8 score) [40]. In Kato’s study, the CONUT score was not associated with postoperative pancreatic fistula, Clavien-Dindo grade, or postoperative duration of hospitalisation [39].
Conclusions
A review of the literature shows that there are a lot of reports regarding the impact of nutritional status on the incidence of postoperative complications in patients undergoing gastrectomy and colorectal surgery. In the field of pancreatic surgery, most studies focus on the influence of nutritional status on short-term outcome in patients following pancreaticoduodenectomy. There are a few reports on the influence of nutritional status in patients following distal pancreatectomy. Therefore, research in this field is needed and should be continued.
In conclusion, nutritional status significantly influences the incidence of postoperative complications in patients undergoing distal pancreatectomy. Assessment of nutritional status using PNI calculation should be the standard management of patients before surgical treatment.