Introduction
Inflammatory bowel disease (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC), is a relapsing-remitting chronic gastrointestinal tract disorder, which is associated with a number of extraintestinal manifestations. The most common systemic complication and extraintestinal manifestation of IBD is anaemia. The prevalence of anaemia reported in systematic reviews varied between 6% and 74% of patients with IBD [1–3]. This very wide range of anaemia frequency is due to the differences in criteria used to define anaemia and the investigated population (e.g. in-patients and out-patients, patients’ sex and age, IBD type). Other factors of variation in the prevalence of anaemia include the time of assessment – at diagnosis or during the course of IBD, the disease activity, and treatment.
Anaemia has a known significant negative impact not only on health-related quality of life, but also on the ability to work and cognitive function, and it is a comorbid condition that is associated with other complications or even death [4–6]. In several studies, anaemia was reported to be the cause of increased need for surgery in IBD patients, hospital admissions, and length of hospitalisation [7]. All of these factors lead to increases in healthcare costs.
The pathogenesis of anaemia is multifactorial. The most common types of anaemia in patients with IBD are iron deficiency caused by chronic blood loss, reduced iron absorption, or malnutrition. The second cause is anaemia of chronic disease, connected with negative impact of pro-inflammatory cytokines on erythropoiesis and shortened red blood cell survival. Vitamin B12 and folic acid deficiencies, use of potentially myelotoxic drugs, and haemolysis may also contribute [8–11].
The detection and treatment of anaemia in IBD are essential in the management of this disease. Most important is the detection and treatment of anaemia in patients at the moment of IBD diagnosis, because it may influence the course of the disease. The main aim of this study was to determinate the prevalence, risk factors, and aetiology of anaemia in adult patients at the time of IBD diagnosis.
Aim
The main aim of this study was to assess the clinical and demographic profile of newly diagnosed IBD patients.
Material and methods
This was a retrospective, single-centre study covering a period of seven years. We included 136 newly diagnosed, hospitalised patients with IBD. The diagnosis of IBD was confirmed by the combination of standard clinical, radiological, endoscopic, and histopathological criteria. Analysed data at the time of diagnosis included patient age, sex, laboratory tests, endoscopic and radiological examination, length of stay, and the course of hospitalisation. The type and localisation of the disease were evaluated using the Montreal classification [12]. Laboratory parameters included levels of haemoglobin (HGB), haematocrit (HCT), red blood cells (RBC), mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH), mean corpuscular haemoglobin concentration (MCHC), C-reactive protein (CRP), albumin, and serum iron concentration were recorded for each patient.
According to the definitions from the World Health Organisation (WHO), anaemia was defined as a decline in blood haemoglobin to a concentration of < 12 g/dl in nonpregnant women and < 13 g/dl in men. Mild anaemia was characterised by a haemoglobin level of 11–13 g/dl in males and 11–12 g/dl in females, moderate anaemia to a haemoglobin of 8 g/dl or more, but less than 11 g/dl in both sexes. Haemoglobin level < 8 g/dl in both genders was considered as severe anaemia [13].
Statistical analysis
Measured values were expressed as mean ± SE. Significance of differences between studied groups was calculated by Student’s t-test, Mann-Whitney U test, and χ2 test. P-values less than 0.05 were considered statistically significant. All statistical calculations were performed using Statistica 13 for Windows (StatSoft, Poland).
Results
During the analysed time period, we included 136 newly diagnosed IBD patients in the study: 61 (44.85%) with Crohn’s disease and 75 (55.15%) with ulcerative colitis. There were no significant differences related to gender in UC (49.3% vs. 50.7%; p > 0.05); however, female predominance was observed in patients with CD (62.3% vs. 37.7%; p = 0.01). The mean age of the patients with IBD at the time of diagnosis was 39.9 ±17.6 years (range: 18–86 years) with no significant differences between men and women both in CD and CU (Table I).
Table I
Demographics and clinical characteristic of newly diagnosed patients with IBD
| Parameter | IBD overall | Crohn’s disease | Ulcerative colitis | P-value* | |
|---|---|---|---|---|---|
| Gender | 136 | 61 | 75 | 0.090 | |
| Male | 60 (44.1) | 23 (37.7) | 37 (49.3) | 0.011 | |
| Female | 76 (55.9) | 38 (62.3) | 38 (50.7) | > 0.05 | |
| Age [years] Mean ± SD (range) | 39.9 ±17.6 (18–86) | 37.8 ±14.6 (18–79) | 41.6 ±19.7 (18–86) | 0.555 | |
| Male | 38.2 ±17.8 (18–84) | 34.3 ±16.4 (18–84) | 40.6 ±18.4 (18–79) | 0.277 | |
| Female | 41.2 ±17.5 (18–86) | 39.9 ±13.2 (20–68) | 42.5 ±21.0 (18–86) | 0.909 | |
| Length of hospitalisation [days] Mean ± SD (range) | 7.54 ±4.27 (1–25) | 7.15 ±3.52 (1–19) | 7.86 ±4.79 (1–25) | 0.469 | |
| Male | 7.44 ±3.88 (1–22) | 6.78 ±2.59 (1–14) | 7.84 ±4.46 (1–22) | 0.316 | |
| Female | 7.62 ±4.58 (1–25) | 7.36 ±3.99 (1–19) | 7.88 ±5.13 (1–25) | 0.836 | |
| Disease location (%) | L1 | – | 7 (13.5) | – | – |
| L2 | – | 10 (19.2) | – | – | |
| L3 | – | 35 (67.3) | – | – | |
| L4 | – | 0 (0) | – | – | |
| Disease behaviour (%) | B1 | – | 32 (61.5) | – | – |
| B2 | – | 11 (21.2) | – | – | |
| B3 | – | 9 (17.3) | – | – | |
| Disease extension (%) | E1 | – | – | 5 (9.4) | – |
| E2 | – | – | 20 (37.7) | – | |
| E3 | – | – | 28 (52.8) | – |
L – disease location: L1 – ileal disease, L2 – colonic disease, L3 – ileocolonic disease, L4 – upper gastrointestinal type; B – disease behaviour: B1 – non-stricturing, non-penetrating type, B2 – stricturing disease, B3 – penetrating disease; E – disease extension: E1 – proctitis, E2 – left-sided disease, E3 – extended colitis.
Figure 1
The prevalence of anaemia in patients with Crohn’s disease (CD) for the different age groups
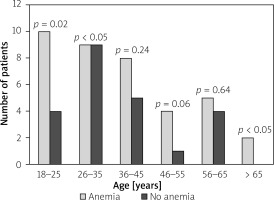
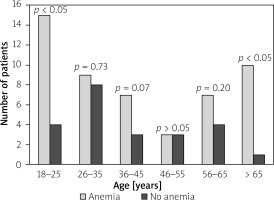
Figure 2
The prevalence of anaemia in patients with ulcerative colitis (UC) for the different age groups
In CD newly diagnosed patients, an ileocolonic disease was observed most commonly – in 35 (67.31%) patients (p < 0.05). An ileal CD was found in seven (13.46%) patients while colonic involvement was seen in 10 (19.23%) patients. Regarding disease behaviour we observed a predominance of non-stricturing, non-penetrating pattern of CD – 32 (61.54%) patients (p < 0.05). A stricturing disease was present in 11 (21.15%) CD patients, and the remaining 9 (17.31%) patients had a penetrating type. For UC, the most frequent was extensive and left-sided colitis, with slight predominance of extensive colitis (28 (52.83%) vs. 20 (37.74%), p = 0.120). A proctitis was diagnosed only in 5 (9.43%) UC patients (Table I).
Anaemia at the time of IBD diagnosis was detected in 89 (65.4%) patients; its frequency was slightly higher in patients with UC than in patients with CD (51 (57.3%) patients vs. 38 (42.7%) patients; p = 0.052). Female patients were more frequently anaemic than males in the whole IBD group (59.6% vs. 40.4%; p = 0.001) (Table II). The mean age of anaemic patients and patients without anaemia did not differ significantly (40.9 ±19.2 vs. 37.7 ±13.5; p = 0.89). However, when the age-stratified prevalence of anaemia at the time of IBD diagnosis was estimated for the age strata 18 to > 65 years we noticed that patients between 18 to 25 years old and those > 65 years old had higher risk of anaemia than patients between 26 and 65 years old for both CD and CU. For the age strata 18 to 25 years old the prevalence of anaemia was 79.0% (p < 0.05) for CU and 71.4% (p = 0.02) for CD. For patients > 65 years old anaemia was detected in 90.9% (p > 0.05) of cases for UC and 100% (p < 0.05) for CD (Figures 1, 2).
Table II
Characteristics of patients with and without anaemia at the time of IBD diagnosis
[i] L – disease location: L1 – ileal disease, L2 – colonic disease, L3 – ileocolonic disease, L4 – upper gastrointestinal type; B – disease behaviour: B1 – non-stricturing, non-penetrating type, B2 – stricturing disease, B3 – penetrating disease; E – disease extension: E1 – proctitis, E2 – left-sided disease, E3 – extended colitis.
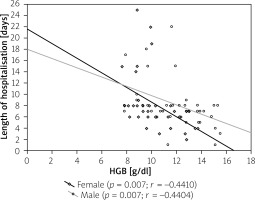
In the analysed population the hospitalisation time was comparable in both types of IBD (7.15 ±3.52 days for CD vs. 7.86 ±4.79 days for UC; p = 0.47) with no differences related to gender (Table I). However, when we compare anaemic patients and patients with no anaemia we noticed that anaemia significantly increased the length of stay (7.95 ±3.8 days vs. 5.88 ±2.7 days for CD; p = 0.02 and 9.02 ±5.0 days vs. 5.00 ±2.4 days for UC; p < 0.05) (Table II). The correlations between haemoglobin level and time of hospitalisation are presented in Figures 3 and 4.
Figure 3
Correlation between length of hospitalisation and haemoglobin (HGB) levels in Crohn’s disease (CD) patients
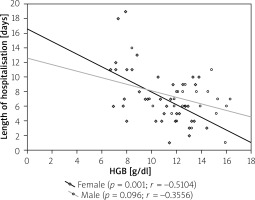
Figure 4
Correlation between length of hospitalisation and haemoglobin (HGB) levels in ulcerative colitis (UC) patients
Anaemia was more frequent in CD patients with ileocolonic involvement compared to other types of disease location (70.96% vs. 56.52%, respectively; p = 0.03). Similarly, patients with a penetrating and stricturing disease behaviour were more commonly anaemic than with non-penetrating and non-stricturing types (30.0% vs. 9.09% for stricturing CD; p < 0.05 and 26.67% vs. 4.55% for penetrating CD; p < 0.05) (Table II). The prevalence of anaemia at the time of diagnosis for UC patients increased with disease extension; for extensive colitis anaemia was diagnosed in 64.71% compared to 31.57% of non-anaemic patients (p < 0.05) (Table II).
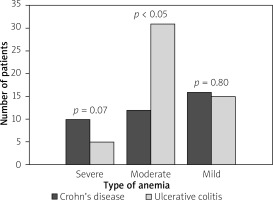
In newly diagnosed IBD patients overall moderate anaemia was most frequent (48.32%), followed with mild anaemia (34.83%). Severe anaemia was diagnosed only in 15 (16.85%) patients. However, when we analysed CD patients and UC patients we found that there was no significant differences in the type of anaemia in CD patients (p > 0.05), while in UC patients moderate anaemia dominated (31 patients – 60.8%; p < 0.05) (Figure 5). The mean HGB level in CD patients was similar to patients with UC for both sexes (for men: 11.5 ±1.3 g/dl vs. 10.6 ±1.7 g/dl; p = 0.084; for women: 9.2 ±1.7 g/dl vs. 9.5 ±1.1 g/dl; p = 0.465) (Table III).
Table III
Haematological profile at the time of diagnosis in anaemic patients with IBD
| Parameter | IBD overall | Crohn’s disease | Ulcerative colitis | P-value* | |
|---|---|---|---|---|---|
| HGB, mean ± SD (range) [g/dl] | Male | 10.9 ±1.6 (7.8–12.8) | 11.5 ±1.3 (8.4–12.7) | 10.6 ±1.7 (7.8–12.8) | 0.084 |
| Female | 9.4 ±1.4 (6.7–11.9) | 9.2 ±1.7 (6.7–11.9) | 9.5 ±1.1 (7.6–11.8) | 0.465 | |
| RBC, mean ± SD (range) [T/l] | Male | 4.03 ±0.65 (3.02–5.35) | 4.44 ±0.54 (3.31–5.35) | 3.79 ±0.60 (3.02–5.01) | 0.003 |
| Female | 3.57 ±0.50 (2.32–4.73) | 3.59 ±0.55 (2.32–4.73) | 3.55 ±0.47 (2.59–4.47) | 0.786 | |
| HCT, mean ± SD (range) (%) | Male | 33.6 ±4.5 (24.0–42.1) | 35.6 ±3.5 (26.7–39.2) | 32.4 ±4.7 (24.0–42.1) | 0.026 |
| Female | 29.3 ±4.0 (21.7–37.7) | 29.0 ±4.7 (21.7–36.5) | 29.7 ±3.2 (23.8–37.7) | 0.543 | |
| MCV, mean ± SD (range) [fl] | Male | 83.7 ±5.8 (70.1–98.6) | 80.6 ±6.3 (70.1–90.1) | 85.5 ±4.8 (78.6–98.6) | 0.012 |
| Female | 82.6 ±7.5 (63.3–97.3) | 81.0 ±7.9 (63.3–93.5) | 84.0 ±7.0 (70.9–97.3) | 0.148 | |
| MCH, mean ± SD (range) [pg] | Male | 27.3 ±2.5 (20.6–33.1) | 26.2 ±2.8 (20.6–30.5) | 27.9 ±2.2 (24.0–33.1) | 0.046 |
| Female | 27.6 ±9.4 (18.4–90.7) | 25.8 ±3.6 (18.4–31.8) | 29.2 ±12.4 (21.9–90.7) | 0.254 | |
| MCHC, mean ± SD (range) [g/dl] | Male | 32.5 ±1.3 (29.0–34.8) | 32.4 ±1.4 (29.0–33.9) | 32.6 ±1.4 (29.0–34.8) | 0.645 |
| Female | 32.0 ±1.6 (28.4–35.1) | 31.8 ±1.8 (28.4–34.9) | 32.1 ±1.4 (29.7–35.1) | 0.383 | |
| WBC, mean ± SD (range) [g/l] | Male | 9.5 ±3.9 (4.0–19.4) | 9.3 ±3.3 (4.8–18.1) | 9.6 ±4.2 (4.0–19.4) | 0.869 |
| Female | 9.3 ±4.1 (3.9–24.5) | 8.4 ±2.8 (3.9–13.3) | 10.2 ±4.9 (3.9–24.5) | 0.285 | |
| PLT, mean ± SD (range) [g/l] | Male | 353.6 ±131.3 (57.0–727.0) | 374.3 ±148.5 (131.0–727.0) | 340.9 ±121.5 (57.0–608.0) | 0.479 |
| Female | 384.0 ±185.3 (89.0–1224.0) | 404.2 ±221.0 (89.0–1224.0) | 363.7 ±142.8 (142.0–641.0) | 0.839 |
Iron deficiency among anaemic IBD patients was found in 69 cases, which represents a prevalence of 77.53% in this population and was more often associated with UC than with CD (59.42% vs. 40.58%; p = 0.027). Bivariate analyses demonstrated a higher proportion of iron deficiency in anaemic women with CD compared to anaemic men with CD (19 (67.86%) vs. 9 (32.14%); p = 0.008); however, we found no significant association between gender in UC (22 (53.66%) in women vs. 19 (46.34%) in men; p = 0.510). The mean iron level among CD patients with accompanying anaemia was 32.44 ±28.84 μg/dl in men and 26.11 ±26.80 μg/dl in women (p = 0.325), while in anaemic patients with UC it was 26.26 ±14.55 μg/dl in men and 28.45 ±20.40 μg/dl in women (p = 0.325).
In most cases vitamin B12 levels were normal, and in one case it was higher than normal (1808.0 pg/ml). No vitamin B12 deficiency was found in the examined group. Considering the MCV and MCH levels the most common type of anaemia in patients with UC was normocytic normochromic anaemia (60.78%; p < 0.05). Less frequently: microcytic hypochromic anaemia (19.61%) and normocytic hypochromic anaemia (17.65%). Among patients with CD we noticed slight predominance of normocytic normochromic anaemia compared to microcytic hypochromic anaemia; however, this difference was not statistically significant (47.37% vs. 36.84%; p = 0.320).
In the analysed population 13 (14.61%) anaemic patients needed blood transfusion – 8 (61.54%) for CU vs. 5 (38.46%) for CD; p = 0.246). There were no significant differences between men and women (p = 0.054). The average amount of transfused blood products was 2.0 ±0.53 U for UC and 1.60 ±0.55 U for CD (p = 0.341). Forty-one (46.07%) IBD patients with anaemia were receiving intravenous iron supplementation – iron (III) isomaltoside or ferric hydroxide sucrose complex. Iron supplementation was more often required for patients with UC than for those with CD – 63.41% vs. 36.59% (p = 0.016).
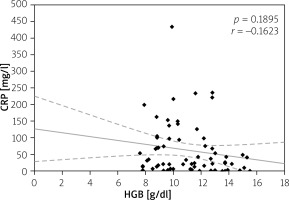
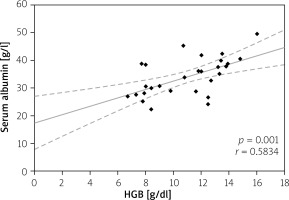
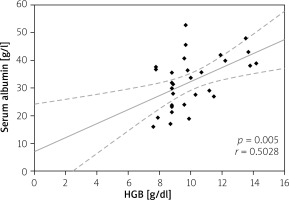
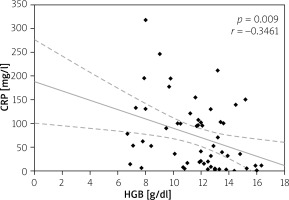
Elevated serum CRP level > 5 mg/l was considered as disease activity level, and it was not significantly different in all CD and UC patients (73.80 ±71.80 mg/l vs. 62.93 ±78.70 mg/l; p = 0.235); however, in anaemic patients its value was higher than in non-anaemic patients (91.65 ±75.84 mg/l vs. 48.96 ±58.64 mg/l for CD (p = 0.017) and 76.63 ±87.54 mg/l vs. 27.57 ±28.14 mg/l (p = 0.027) for UC). Moreover, we analysed the correlation between serum CRP and HGB levels. Among CD patients the CRP level showed a negative correlation with HGB level (p = 0.002), while in patients with UC there was also a negative correlation, but it did not reach statistical significance (p = 0.07) (Figures 6, 7). In our study hypoalbuminaemia was diagnosed in 34.56% of patients (37.7% in CD patients and 32.0% in UC patients) with similar medium level in both CD and CU (34.09 ±6.85 g/l vs. 32.6 ±9.53 g/l; p = 0.498). We also noticed positive correlation between serum albumin and HGB levels in both IBD types (p < 0.05) (Figures 8, 9).
Figure 6
Correlation between C-reactive protein (CRP) and haemoglobin (HGB) levels in Crohn’s disease (CD)
Discussion
The incidence of UC in Europe is about 10 cases per 100,000 population a year, and CD from 0.9 to 11.4 cases per 100,000 people annually, and in the past few decades it has increased [14–16]. This epidemiology findings are similar to available studies even from non-European countries [17]. However, in Poland, analyses of epidemiological data related to IBD, especially newly diagnosed patients, are still insufficient. This lack of current long-term studies, mainly prospective, which provides more reliable data, means that the epidemiological trends in newly diagnosed IBD cannot be clearly determined. In one retrospective study on the Polish population the rates of hospitalisation for IBD and time trends in the past two decades were analysed [2]. That study showed that in each consecutive year the rates of hospitalisation were higher than during the previous year, and they were a few times higher for UC than for CD. Jakubowski et al. suggested that their findings can be connected to the increasing prevalence and incidence of IBD in Poland. However, in our 7-year period of study the occurrence of UC was similar to CD. Patients who were newly diagnosed with CD were more likely to be female, and there were no sex differences in those patients with UC, which correspond with other published studies conducted on the general population of IBD patients [18–20].
Several studies showed that anaemia affects most patients with IBD [1–3]. However, those analyses refer to all hospitalised patients, not only to those at the time of diagnosis. The connection between anaemia and IBD has been intensively researched, but data at the time of IBD diagnosis are still insufficient. In a similar study of a European population of newly diagnosed IBD patients, but of 1-year observation only, anaemia was present in 41.2% of patients [21]. In our report the percentage of anaemia was 65.4% in newly diagnosed hospitalised IBD patients, which is slightly higher and probably reflects the longer observation time.
In contrast to our study, in which there was no difference regarding the frequency of anaemia between CD and UC patients, Burish et al. and Lucendo et al. reported higher risk of anaemia in CD than UC at the time of diagnosis [17, 21]. The findings of those researchers are similar to the general trend in the IBD population.
The diagnosis of IBD can be made at any age. We found that newly diagnosed hospitalised IBD patients between 18 to 25 years old and those over 65 years old had higher risk for anaemia than those between 25 and 65 years old for both types of this disease. Similar data are available from different studies, and this presents a trend for higher risk of anaemia in younger and older patients both at the time of diagnosis and during the course of the disease [21, 22]. One of the common findings in previous studies, which represents a worldwide trend, was female predominance among anaemic IBD patients [19, 20]. We also demonstrated that the frequency of anaemia was higher in women both with CD and UC in newly diagnosed hospitalised IBD patients.
To find other determinants of this common complication we analysed the correlation between the occurrence of anaemia and IBD location, extension, and behaviour. In our study, the predominant form of UC at the time of diagnosis was extensive, left-sided colitis, which is similar to other reports [20, 23]. In addition, in other studies as well as ours, patients with more extensive form of UC were more frequently anaemic. In CD, some studies of newly diagnosed patients show the prevalence of ileal disease [24, 25], whereas another analysis found that the ileocolonic type was predominant [23]. Our findings agree with the second group of researchers, who also found that the most common disease behaviour is non-stricturing and non-penetrating type [23]. The occurrence of anaemia in CD was also connected with disease activity and was diagnosed mostly in patients with ileocolonic involvement and penetrating and stricturing disease.
During the 7-year time period we analysed the mean length of hospitalisation stay, and we did not find any differences regarding IBD type and patient gender. The mean length of stay of our IBD patients was similar to that presented by Jakubowski et al. They showed that the average length of stay declined from 10.2 to 7.9 days for CD and from 8.5 to 6.5 days for UC, and it was progressively shorter in each year of analysis [2]. Slightly longer time of hospitalisation was observed by Bernstein et al. [7]. However, both studies did not analyse patients at the time of IBD diagnosis, and they did not study the influence of anaemia on length of stay. In our study we clearly proved that anaemia is one of the factors extending time of hospitalisation in newly diagnosed IBD patients.
Analysing the data of anaemic IBD patients in our study we can confirm that anaemia has a multifactorial aetiology. In our study, as well as that of Lee et al., normocytic normochromic type of anaemia was the most common [26]. However, studying CRP levels we noticed that they were higher in CD patients, and we found a strong negative correlation between CRP and HGB levels in contrast to UC patients, in whom this correlation was not statistically significant. Previous studies confirm the coincidence between occurrence of anaemia and increased inflammatory parameters [27, 28]. Analysing the above this suggests the predominance of anaemia of chronic disease in CD patients, and of iron deficiency anaemia in UC patients, which is the same as in other studies [23, 28, 29]. Moreover, analysing serum iron levels, we showed that iron deficiency is more commonly associated with UC that with CD. The prevalence of iron deficiency in all newly diagnosed hospitalised IBD patients was 77.53%. In several studies in patients with IBD serum iron deficiency was also detected in over half of the studied population [22, 30], which suggests the important role of this aetiology of anaemia in IBD patients. However, the estimation of the prevalence of types anaemia still requires more precise analyses.
In our study severe anaemia was present in 6.51% of patients, which is similar to other studies [21, 31]. Patients with CD more often had severe anaemia than did those with UC, in contrast to mild and moderate anaemia where patients with UC dominate over CD. This finding may suggest that chronic blood loss and inflammation are more severe in CD than in UC.
Low serum albumin level prevalence was not different between CD and UC patients in our research. Hypoalbuminaemia has a strong impact on anaemia occurrence, which we confirmed in our study, showing a positive correlation between serum albumin and HGB levels. In both types of IBD anaemia was more frequent in patients with reduced albumin level. Khan et al. analysed in newly diagnosed UC patients the relation of lower albumin levels and changes in HGB levels during the time of disease. They found that lower albumin level predicts changes in HGB level from no-anaemia or mild anaemia to moderate or severe anaemia over the natural course of disease [31]. It shows the importance of adequate diagnosis and treatment of anaemia and its aetiology. In our research nearly half of the anaemic patients received intravenous iron treatment. Several studies have shown that intravenous supplementation in patients with active disease is more efficient than oral iron usage [32, 33].
The main limitations of this study were its retrospective character and the lack of additional laboratory tests, which could help in differential diagnosis of anaemia type. Despite these limitations, our study provides new epidemiological data and show factors connected to anaemia occurrence in newly diagnosed, hospitalised IBD patients. Our study is one of the few studies evaluating the incidence, severity, and risk factors of anaemia at the moment of IBD diagnosis in hospitalised patients.
Conclusions
Anaemia is a frequent complication of IBD, not only during the long-term course of disease, but also at the disease onset. Our findings suggest that female sex and disease activity are strong determinant factors connected with anaemia. Occurrence of this extraintestinal manifestation of IBD significantly extends the time of hospitalisation.