Purpose
Prostate cancer causes significant morbidity and is the second most common cause of cancer-specific mortality in men in the United States [1]. In the era of prostate-specific antigen (PSA) testing, patients are frequently diagnosed with localized, low- and intermediate-risk disease [2,3]. Management options include radical prostatectomy (RP), external beam radiotherapy (EBRT), brachytherapy (BT), or active surveillance (AS). Despite an overall decline in the use of BT alone or as a boost in combination with EBRT since the early 2000s, monotherapy using either high-dose-rate (HDR) or low-dose-rate (LDR) brachytherapy remains one of the most cost-effective options for patients with localized prostate cancer compared to other modalities [4,5,6,7].
Monotherapy with either HDR or LDR brachytherapy both represents efficacious, high-value options for treating localized prostate cancer. However, no prospective, randomized data are currently available comparing these two methods in either the context of monotherapy or as a boost to EBRT. Adopted into clinical practice first, the collective LDR experience includes more patients with greater follow-up duration compared with HDR [8,9,10]. However, initial institutional outcomes with HDR monotherapy suggest encouraging rates of disease control, potentially improved urethral and rectal dosimetry, and favorable acute toxicity profiles [11,12,13,14,15,16].
Consequently, the choice between HDR vs. LDR is likely dependent on a variety of factors, including patient characteristics, physician expertise, and treatment facility preference, although data informing these decisions are sparse. We therefore sought to determine treatment patterns, clinical outcomes, and patient factors influencing the choice between LDR and HDR monotherapy in a modern cohort of patients with low- and intermediate-risk prostate cancer using a large hospital-based registry, the National Cancer Database (NCDB).
Material and methods
We queried the 2014 participant user files of the NCDB for patients with prostate cancer diagnosed in 2004-2014, treated with brachytherapy as their sole method of radiation therapy. NCDB is a joint program of the American College of Surgeons and the American Cancer Society that provides data available on patients diagnosed at Commission on Cancer (CoC) accredited cancer centers, capturing approximately 70% of all newly-diagnosed cancer annually in the United States [17]. All variables used in the analysis are described in the participant user files (PUF) data dictionary version 2014, and any derivations are outlined in the tables. We included patients with localized low- or intermediate-risk prostate cancer, specifically those with stage ≤ cT2c, Gleason score ≤ 7, and/or PSA < 20. We excluded patients who received external beam radiation or RP and patients who had node-positive or high-risk prostate cancer. We included patients who receive hormone therapy [10].
Descriptive statistics were applied to analyze patterns of use of HDR compared to LDR. Relationships between HDR vs. LDR use and patient demographics and disease characteristics (race/ethnicity, age, year of diagnosis, PSA, Gleason score, insurance, T stage, facility type and location, urban/rural/metro residence, Charlson-Deyo comorbidity index, hormone therapy receipt, zip-code level income, and education) were assessed using multivariable logistic regression. Equality of HDR vs. LDR use by covariates with more than 2 levels was assessed using the multivariable Wald test.
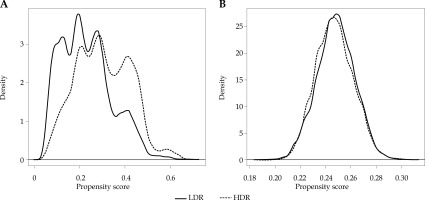
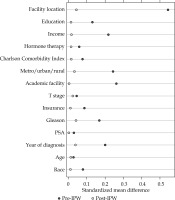
Survival among HDR and LDR recipients was assessed using Kaplan-Meier curves, log-rank tests, and Cox proportional hazards regression. To account for potential selection bias in the HDR and LDR groups, we included the aforementioned covariates in the proportional hazards model and performed Kaplan-Meier and log-rank tests with inverse probability weights (IPW) derived from the predicted values of a multivariable logistic regression model with the same set of covariates. The success of covariate balance was evaluated by standardized mean differences and kernel density plots.
Adequacy of the proportional hazards’ assumption was formally tested comparing Schoenfeld residuals against transformed time, and covariates with non-proportional hazards were removed and instead used as strata. Analyses were performed in R 3.3.2 using the survival and IPW survival packages, and figures were generated using the ggplot2, lattice, and latticeExtra packages. All p-values were 2-sided if applicable, and p < 0.05 was considered statistically significant.
Results
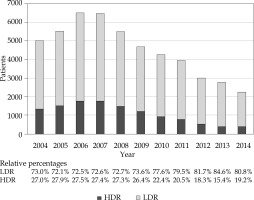
We identified 37,863 LDR and 12,463 HDR recipients who met the aforementioned inclusion and exclusion criteria. Patient characteristics are reported in Table 1. Median follow-up was 70.3 (range, 0-143.2). Overall, the application of BT monotherapy decreased over the study period, with HDR monotherapy use declining relative to LDR (Figure 1). In 2004, 27.0% of monotherapy cases were HDR compared to 19.2% in 2014 (odds ratio [OR] 0.60, p < 0.001).
Table 1
Patient and treatment related characteristics
On multivariable analysis (Table 2), black patients were more likely to receive HDR than whites (OR: 1.181, 95% CI: 1.113-1.253, p < 0.001). Receipt of HDR varied significantly by age (p-value for difference = 0.028), where older patients were more likely to receive HDR (OR of HDR receipt relative to patients < 50 years: 0.944 for 50-59, 1.033 for 60-69 years, 1.041 for 70-79 years, and 1.125 for > 80 years). Those diagnosed after 2010 were less likely to receive HDR than those diagnosed earlier (OR: 0.546, 95% CI: 0.498-0.599, p < 0.001). Patients with stage cT2b disease were less likely to receive HDR (OR: 0.842, 95% CI: 0.734-0.965, p = 0.013), whereas patients with stage cT2c disease were more likely to receive HDR (OR: 1.188, 95% CI: 1.055-1.339, p = 0.005) relative to those with cT1c (reference) or cT2a disease. Receipt of HDR treatment was more likely in an academic facility (OR: 1.684, 95% CI: 1.605-1.766, p < 0.001) relative to a non-academic facility. Metropolitan residents were more likely than rural (reference) and urban residents to receive HDR (OR: 1.36, 95% CI: 1.142-1.618, p = 0.001). Relative to those with a Charlson-Deyo comorbidity index of 0, those with an index of 1 were less likely to receive HDR (OR: 0.929, 95% CI: 0.868-0.994, p = 0.032). Recipients of hormone therapy were less likely to receive HDR than non-recipients (OR: 0.917, 95% CI: 0.869-0.968, p = 0.002). Receipt of HDR varied significantly by income (p-value for difference < 0.001) and education (p-value for difference < 0.001), where residents of the highest-earning (OR: 1.074) or most highly-educated (OR: 1.208) zip-codes were more likely to receive HDR than those in lower-earning (mid-low, specifically) and less-educated (low, specifically) zip-codes. Receipt of HDR also varied significantly by region (p < 0.001).
Table 2
Multivariable logistic regression analysis of association between HDR vs. LDR and patient characteristic
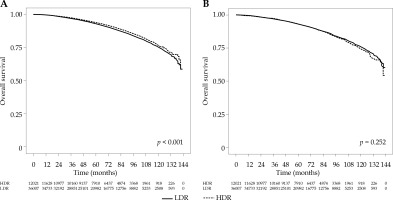
The survival curves for HDR and LDR recipients are given in Figure 2A. Overall survival (OS) at 5- and 10-years was 92.9% and 75.3% for LDR and 93.9% and 76.6% for HDR, respectively, with HDR recipients having significantly better survival (p < 0.001). After IPW adjustment to account for potential selection bias (see Figures 3 and 4), the difference was non-significant (p = 0.252), with estimated 5- and 10-year OS of 93.1% and 75.9% for LDR and 93.2% and 74.2% for HDR, respectively (Figure 2B). In the proportional hazard regression analysis, the receipt of HDR vs. LDR was not significantly associated with survival (HR: 0.82, 95% CI: 0.28-2.406, p = 0.718). Furthermore, there were no patient or disease characteristics that had varied survival based on the type of brachytherapy received (p > 0.062).
Discussion
Our study of this large patient cohort reveals that the overall use of brachytherapy as monotherapy for low- and intermediate-risk prostate cancer has been steadily declining in recent years. We found that relative to LDR, the proportion of patients treated with HDR monotherapy exhibited an overall decline as well. We found that the receipt of HDR vs. LDR monotherapy differs significantly by nearly every patient factor, sociodemographic, and disease-specific. Our work identified that HDR monotherapy was more likely in patients who were black, were older, and lived in zip-codes associated with higher income and education; in patients who had fewer comorbidities, had stage cT2c disease, and had not received hormone therapy; and in patients treated in academic facilities, facilities in metropolitan areas, or facilities in coastal or Northeastern locations. Furthermore, our results suggest that the overall survival is similar for patients treated with HDR and LDR brachytherapy.
An overall decline in brachytherapy for prostate cancer, both as a boost with EBRT and as monotherapy, has been previously reported, both within the percent of patients receiving any form of radiotherapy, declining from 45% to 38% from 2005 to 2009, and as a percent of those receiving any definitive therapy, declining from 17% to 8% from the peak year of 2002 to 2010 [18,19]. Furthermore, the percent of brachytherapy monotherapy among all types of prostate cancer management strategies, including those without definitive treatment, has declined from 12.1% in 2004 to 4.6% in 2014, with stable trends in EBRT (22.5% in 2004 to 23.4% in 2014) and further decreases in EBRT + BT (5.3% in 2004 to 3.4% in 2014) [20]. These decreasing rates of brachytherapy are attributable to several factors, such as the increased adoption of newer technologies like stereotactic body radiotherapy, maturing data for moderately-hypofractionated EBRT, reimbursement incentives, and reduced physician training in brachytherapy. For instance, increases in the use of IMRT and robotic surgeries have coincided with a decline in the number of brachytherapy cases [21,22,23,24].
Regarding newer technologies, Martin and colleagues identified the peak year of prostate brachytherapy use to be 2002 before a general decline, directly preceded by the first report of robot-assisted laparoscopic prostatectomy and FDA approval of CyberKnife for prostate cancer, both in 2001 [19]. Finally, the exposure to brachytherapy procedures has been reportedly declining in resident training programs, decreasing the pool of experienced brachytherapists who are willing to perform the procedure [25,26].
Observing that rates of brachytherapy are decreasing is surprising, as brachytherapy for low- to intermediate-risk prostate cancer has been demonstrated to have equivalent or superior outcomes relative to other options and is thought to be to be the least costly active treatment option [4,27,28,29]. In one analysis, median cost of SBRT was $27,145 compared to $17,183 for brachytherapy, $37,090 for IMRT, and $54,706 for proton beam therapy (p < 0.001). However, another study did suggest that SBRT might be slightly more cost effective than LDR brachytherapy [30,31].
Our findings that HDR monotherapy use is decreasing relative to LDR are also surprising, given the potential radiobiological advantages of HDR, and encouraging disease control and quality-of-life outcomes to date [11,12,13,14,15,16,32]. One explanation may be that HDR may be less convenient to the patient, since LDR typically involves a single outpatient procedure, whereas HDR often requires multiple implants and potential hospital admission; however, further maturation of outcomes data for single-fraction HDR may mitigate these differences [12]. The price of HDR may also compare favorably relative to LDR. It has been suggested that single-fraction HDR monotherapy is least expensive ($9,850), followed by laparoscopic surgery ($11,098), then open radical prostatectomy ($13,829), and LDR brachytherapy ($13,893), with combined EBRT and BT being the most expensive ($18,819); however, another study suggested that HDR may become more expensive than LDR ($6,869), with increasing fractions (single fraction: $5,582; multifraction: $9,538) [33,34]. Interestingly, the use of HDR brachytherapy has been shown to be increasing in Europe, contrary to the findings of our current study in a large, United States-based cohort [35]. No randomized controlled trials comparing LDR and HDR BT as monotherapy have been conducted, though outcomes of the two appear similar upon synthesis of the available data [36]. Our retrospective study also found no differences in survival between the two modalities, which is unsurprising, given the low- and intermediate-risk patients included in this analysis.
Despite the strengths of our study, it is affected by several limitations inherent to its design. As this is a retrospective database analysis, our results may be biased or inaccurate due to selection bias, incomplete data, and coding errors. Furthermore, since the NCDB only includes hospitals that are CoC accredited, which tend to be larger and in urban locations with more cancer-related services, we may underrepresent patients who are referred directly for outpatient therapy at non-hospital sites, thus potentially omitting a disproportionate number of patients receiving RT. Furthermore, while we utilized inverse probability weights to address this, a direct comparison of the efficacy of LDR and HDR is difficult due to the retrospective nature of the project, and thus no causal inference can be made. The NCDB includes records from many facilities, and the observations from these facilities may be non-independent; however, a regression model with robust standard errors to account for potential correlation among observations gave virtually identical results (data not shown). Finally, the outcome comparison in this analysis is limited, as the NCDB only reports overall survival information, so we were unable to compare biochemical control and locoregional or distant failure, which may be more useful metrics for low- and intermediate-risk prostate cancer.
Conclusions
We observed an overall decline in the use of brachytherapy monotherapy for the treatment of low- and intermediate-risk prostate cancer. While HDR and LDR monotherapies were associated with comparable long-term survival outcomes, the use of HDR significantly decreased relative to LDR. Furthermore, several patient characteristics were predictive for receipt of HDR versus LDR monotherapy.