INTRODUCTION
Lupus erythematosus is an autoimmune connective tissue disease divided into two forms: systemic (systemic lupus erythematosus – SLE), and cutaneous (cutaneous lupus erythematosus – CLE). Although the skin lesions in SLE and CLE may be the same, patients who do not meet the qualifying criteria for SLE are classified as having CLE [1]. These lesions include acute cutaneous lupus erythematosus (ACLE), subacute cutaneous lupus erythematosus (SCLE), and chronic cutaneous lupus erythematosus (CCLE) which are distinguished by clinical features, duration of skin lesions, and histological features [2, 3]. CCLE may be further divided into subtypes such as discoid lupus erythematosus (DLE), lupus erythematosus profundus (LEP), chilblain lupus erythematosus (CHLE), and lupus erythematosus tumidus (LET) [4].
Similarly, clinical manifestations of psoriasis may differ. The most common form is psoriasis vulgaris, also known as plaque-type psoriasis. It presents as well-demarcated, erythematous plaques covered with a silvery-white scale. The symmetrical distribution of these plaques and their location on the trunk, extensor surfaces of the limbs, and scalp are characteristic features indicative of psoriasis. Other subtypes include pustular psoriasis, guttate psoriasis, inverse psoriasis, and erythrodermic psoriasis [5].
The prevalence of CLE and psoriasis remains uncertain. Additionally, there were insufficient data concerning the correlations between different types of CLE and psoriasis, as well as the specific skin regions affected. Moreover, the treatment strategies utilized for these coexisting conditions were unclear.
It is important to note that there are no systematic reviews in the literature regarding the coexistence of CLE and psoriasis. This article aims to summarize and evaluate the existing literature, providing a comprehensive overview of this topic.
METHODS
Protocol and literature search
The current review was conducted in accordance with the 2020 Preferred Reporting Items for Systematic Reviews and Meta-Analyses literature search extension (PRISMA-S) statement. The literature available on PubMed, EMBASE, Scopus, and Web of Science databases was reviewed using the MeSH terms: “cutaneous lupus erythematosus” and “psoriasis” with no time restrictions. The search terms were combined using the Boolean operator “AND” between the text terms.
Selection of articles
Articles were initially evaluated based on their title, and those identified as potentially relevant, underwent further evaluation through abstract and full-text assessment. Any disagreements regarding article relevance were resolved by the independent author (AKB). Inclusion criteria for data extraction and analysis were based on the following criteria: (a) confirmation of CLE diagnosis through clinical presentations and histopathological examination, and (b) confirmation of diagnosis of psoriasis. Articles inaccessible in the full text were excluded. No language limits were imposed as long as articles could be adequately translated using Google Translate.
Data extraction and analysis
Three authors (IS, LM, HP) conducted a critical review of the included articles and independently extracted the following variables into a Microsoft Excel spreadsheet: age, gender, type of CLE and psoriasis, presence of ANA with specification, hypersensitivity, localization of skin lesions, non-cutaneous symptoms indicative of systemic involvement, accompanying diseases, and treatment. Any discrepancies in the extraction of the variables were resolved through discussion with the independent author (AKB).
Not all patients had data available for every variable. Therefore, percentage results are based on the number of patients with available information for a given feature. Continuous variables such as age were reported as means and ranges.
The data were synthesized using Excel functions and presented in the accompanying tables.
Limitations, quality, and risk of bias
Three authors (IS, HP, LM) independently assessed the methodological quality of the evidence and the risk of bias of the included studies based on previously established criteria for case reports and series. Any discrepancies were resolved through discussion with the fifth author (AKB). It is important to note that the under-recognition and under-publication of CLE and psoriasis may impact the reliability of the presented data, potentially leading to detection and publication biases. Moreover, some reported cases lacked precise descriptions and were not always complete relative to the investigated variables. The heterogeneity in data presentation across different studies posed a limitation during data extraction, possibly leading to misdiagnosis and either overestimation, or underestimation of the number of cases.
RESULTS
Identification of eligible articles
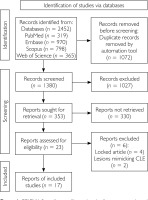
As shown in the PRISMA flow diagram (fig. 1), the initial literature search identified 2,452 articles from various databases (PubMed, EMBASE, Scopus, Web of Science). After removing duplicates (n = 1072) and articles deemed irrelevant based on title and abstract screening (n = 1380), 23 articles underwent full-text evaluation. No additional records were identified through the references from the included articles. Among them, 17 articles fulfilled the eligibility criteria and were included in the synthesis, as detailed in supplementary table S1 [6–22], resulting in a final sample of 17 patients for analysis. Excluded articles (n = 6) are summarized in supplementary table S2 [23–29].
Clinical features
The mean age of all patients was 52 years (range: 22–74 years). The majority were women (12/17; 70.6%) with a mean age of 55.8 years.
The most common type of CLE co-occurring with psoriasis was SCLE (58.8%), followed by drug-induced SCLE (17.6%), DLE (17.6%), and chilblain LE (5.9%). No cases were reported with the coexistence of psoriasis and ACLE, lupus tumidus, lupus profundus, or lupus erythematosus lichen planus (LE-LP) overlap syndrome. Plaque psoriasis was the most common type of psoriasis found in coexistence with CLE, observed in 82.35% of cases, followed by psoriatic arthritis (17.6%) which occurred with plaque psoriasis in 3 reported patients, guttate psoriasis (11.8%), and inverse psoriasis (5.9%). No cases of CLE with erythrodermic psoriasis, pustular psoriasis, nail psoriasis, or palmoplantar psoriasis were identified. Approximately 23.5% of patients with comorbid CLE and psoriasis exhibited hypersensitivity to ultraviolet (UV) radiation. The majority of the patients (82.35%) tested positive for anti-ANA antibodies, 47% had anti-Ro/SSA antibodies, and 5.9% had antihistone antibodies. None of the patients were diagnosed with SLE. CLE lesions were predominantly located on the head, neck, and face areas (62.5%), followed by the chest/abdomen (43.75%), and upper limbs (43.75%). CLE skin lesions were also observed on the back (43.75%) and lower limbs (37.5%). Psoriasis most commonly affected the lower limbs (62.5%), followed by the upper limbs (37.5%), back (31.25%), head-neck-face (18.75%), and chest/abdomen (12.5%). Demographic and clinical features are summarized in tables 1 and 2.
Table 1
Epidemiology and demography of coexistence of CLE and psoriasis
Table 2
Clinical features and presentations
Treatment of CLE
In the management of CLE lesions, the most frequent course of action was the discontinuation of the monoclonal antibody employed in the treatment of psoriasis (37.5%). The hydroxychloroquine (31.25%) and oral glucocorticosteroids (31.25%) were the second most commonly employed medications in the management of CLE in patients who had coexisting CLE with psoriasis. Locally, topical glucocorticosteroids (25%) and photoprotection (25%) were the most commonly used. Methotrexate (12.5%), ustekinumab (12.5%), topical calcineurin inhibitors (6.25%), and colchicine (6.25%) were used less frequently in patients due to CLE.
Treatment of psoriasis
In the treatment of psoriasis in patients with cooccurrence of psoriasis and CLE, topical corticosteroids (31.25%) and methotrexate (31.25%) were the most often used. Furthermore, numerous monoclonal antibodies, such as infliximab (18.75%), ustekinumab (12.5%), secukinumab (12.5%), adalimumab (6.25%), and brodalumab (6.25%), were employed. The treatment of psoriatic lesions in patients with co-occurrence of CLE and psoriasis involved the utilization of calcineurin inhibitors (12.5%), local vitamin D analogues (6.25%), and UVB light (6.25%), PUVA (6.25%), methoxypsoralen (6.25%), the Goeckerman method (6.25%), cyclosporine (6.25%) and in one instance, alefacept was employed (6.25%). Treatment data are summarized in table 3.
Table 3
Drugs used to treat CLE and psoriasis
Accompanying diseases
The most commonly observed comorbidities in patients with coexisting CLE and psoriasis were those associated with metabolic syndrome, including diabetes, hyperlipidaemia, hypercholesterolemia, and arterial hypertension.
In addition to conditions related to metabolic syndrome, patients with concomitant CLE and psoriasis also presented with autoimmune diseases such as Sjögren’s syndrome, endocrine diseases like hypothyroidism, and cardiac diseases including ischemic cardiopathy. Associated disease data are summarized in table 4.
Table 4
Associated diseases
DISCUSSION
Epidemiology, clinical manifestation, and associated diseases
The present study of the co-occurrence of CLE with psoriasis revealed a predominance of female patients (70.6%), with a mean age of 55.8 years, which is consistent with many autoimmune connective diseases [2, 29]. The annual incidence rate of CLE is estimated to range from 2.74 to 4.36 per 100,000 [30], with a higher risk of CLE in African Americans compared to Caucasian populations [2].
Psoriasis, on the other hand, is an inflammatory disease affecting approximately 60 million people worldwide, with a prevalence of up to 5% of the population in Europe [5]. Studies indicate a higher occurrence of psoriasis in women, with the mean age of onset typically earlier in women compared to men. This condition is more frequently diagnosed in high-income countries and aging populations [31], with a higher prevalence among Scandinavian and Caucasian populations compared to African or Asian descent [32].
Psoriasis may extend its impact beyond the skin to affect other organs. Approximately 40% of psoriasis patients develop psoriatic arthritis which increases the risk for metabolic syndrome, which comprises hyperlipidaemia, hypertension, coronary artery disease, type 2 diabetes, and elevated body mass index [32, 33]. Furthermore, systemic inflammation in patients with psoriasis increases the risk of myocardial infarction, stroke, and cardiovascular-related mortality [32, 34]. Studies have also identified a correlation between psoriasis and inflammatory bowel diseases, particularly Crohn’s disease, as well as chronic kidney disease [32, 35]. Some evidence suggests a link between psoriasis and non-alcoholic fatty liver disease (NAFLD) [36].
This systematic review revealed that the most frequently observed comorbidities in patients with concurrent CLE and psoriasis are those related to metabolic syndrome, including diabetes, hyperlipidaemia, hypercholesterolemia, and arterial hypertension. Additionally, Sjögren’s disease, hypothyroidism, and ischemic cardiomyopathy were among the observed comorbidities in patients with coexisting CLE and psoriasis.
Clinical manifestations
In this systematic review comprising 17 cases, typical localization patterns were observed for both psoriasis and CLE. The most prevalent types of CLE occurring in psoriasis were SCLE, followed by druginduced SCLE, DLE, and chilblain LE. Notably, no cases were reported involving the coexistence of psoriasis with ACLE, LE tumidus, LEP, or LE-LP overlap syndrome.
As expected, plaque psoriasis was the most common type of psoriasis observed in coexistence with CLE. Moreover, approximately 23.5% of cases with comorbid CLE and psoriasis exhibited hypersensitivity to UV radiation. The majority of patients tested positive for anti-ANA antibodies. CLE lesions were most frequently found in areas such as the head-neck-face, chest/abdomen, and upper limbs with additional occurrence on the back and lower limbs. Psoriasis predominantly affected the lower limbs, followed by the upper limbs, and the back. The head-neck-face, and chest/abdomen were among the least affected areas.
Pathogenesis of cutaneous lupus erythematosus and psoriasis
CLE is a multifactorial disease influenced by genetic, environmental, and immunopathological factors. Genetic studies have highlighted associations between CLE and genes such as human leukocyte antigen (HLA) subtypes, tumor necrosis factor (TNF-α), and complement promoter variants. Moreover, single nucleotide polymorphisms in interferon regulatory factor 5 (IRF5), cytotoxic T-lymphocyte-associated protein 4 (CTLA4), integrin alpha M (ITGAM), and tyrosine kinase 2 (TYK2) have been identified [2, 37]. The most common environmental factor inducing CLE is UV light exposure [37].
Furthermore, interleukin-23 (IL-23), helper T cells type 17 (Th17), TNF-α, interferons as well as genetics play the most important roles in pathogenesis of psoriasis. TNF-α is a cytokine which promotes inflammation. It is responsible for amplifying of the effects of other cytokines, including IL-17 which influences Th17 cells [5].
One potential common pathway for the pathogenesis of CLE and psoriasis may be the involvement of TNF-α in the pathogenesis of these two conditions. The mechanisms underlying these conditions remain unclear, emphasizing the necessity for further research in this area.
Treatment of CLE preceding psoriasis
Initially, monoclonal antibodies were used to treat psoriasis. Ustekinumab-induced SCLE was managed with topical betamethasone valerate ointment, a tapering course of oral glucocorticosteroids, and hydroxychloroquine. It is important to note the possibility of hydroxychloroquine exacerbating psoriasis. While lupus erythematosus may start to clear, unfortunately, psoriasis may begin to flare [13].
Brodalumab was utilized for psoriasis but resulted in SCLE. Discontinuation of brodalumab led to spontaneous resolution of the SCLE lesions [8]. Similarly, secukinumab induced SCLE after four doses. Upon discontinuation of secukinumab, treatment with hydroxychloroquine and topical clobetasol propionate was initiated [10]. In another case where secukinumab caused SCLE, discontinuation of the drug was followed by treatment with topical methylprednisolone and application of photoprotection (SPF 50) [14]. Infliximab may induce CLE. Treatment of psoriasis with methotrexate and infliximab resulted in the occurrence of SCLE, which was managed by switching from infliximab to guselkumab. Additionally, oral colchicine and a low dose of prednisone were administered to target SCLE [6]. Moreover, infliximab used to treat psoriasis may also lead to lupus-like symptoms characterized by malar rash, arthralgias, and diffuse joint swelling distinct from psoriatic arthritis with positive ANA antibodies. In such cases, treatment involved discontinuation of infliximab and initiation of oral prednisone [18]. Adalimumab therapy has the potential to cause chilblain lupus. In cases of chilblain lupus induced by adalimumab therapy, discontinuation of adalimumab was necessary as a treatment for CLE [9].
For the treatment of pustular psoriasis that appeared initially, the patient received topical triamcinolone, and subsequently, for SCLE which occurred 1 year later, the patient was treated with topical betamethasone, photoprotection, in addition to oral hydroxychloroquine [12]. Sun protection is crucial as sunlight exposure can cause or exacerbate lupus lesions. In 1 reported case, psoriasis was treated with phototherapy resulting in the development of SCLE, which was treated with topical glucocorticosteroids [22]. A treatment regimen involving hydroxychloroquine for lupus control and glucocorticosteroids for local psoriatic lesions has also been documented [17]. The administration of cyclosporine orally was documented once [21]. One of the included case reports does not contain any information regarding the patient’s treatment [20].
Treatment of psoriasis preceding CLE
When CLE first occurred, the patient was typically already on antimalarial or immunosuppressive medications to manage lupus erythematosus. In the case of inverse psoriasis, topical treatments such as calcipotriol with betamethasone dipropionate and pimecrolimus were used, while hydroxychloroquine was orally administered to control SCLE [7]. Although hydroxychloroquine is commonly used in SCLE treatment, it can sometimes trigger severe psoriasis. This scenario was observed when hydroxychloroquine was utilized for SCLE alongside alefacept for psoriasis, with both conditions diagnosed simultaneously [19].
DISCUSSION
There is a report suggesting that ustekinumab might be effective in treating psoriasis concurrent with initial SCLE [11]. In a patient with severe psoriasis and hypertrophic CLE treated with ustekinumab, significant improvement was observed in lupus plaques, along with total clearance of psoriasis [16]. In another case, where DLE appeared first followed by psoriasis, oral methotrexate, topical glucocorticoids, and sun protection were administered [15].
Each case presented unique challenges and required an individualized approach by a dermatologist. Therapeutic options underscore the importance of addressing these two diseases separately in accordance with their treatment guidelines. It is significant to note that UV light exposure can worsen CLE, and hydroxychloroquine may exacerbate psoriasis.
In many cases, the primary treatment for CLE involved discontinuing monoclonal antibodies commonly used to treat psoriasis, which often led to the emergence or exacerbation of CLE lesions. These medications included TNF-α inhibitors such as infliximab and adalimumab as well as IL-17 inhibitors such as secukinumab and brodalumab [6, 8–10, 14, 18]. Additionally, IL-23 inhibitors like ustekinumab have been implicated [11, 13, 14]. This may suggest a potential exacerbation of skin manifestations in CLE with inhibition of these molecules.
As expected, the majority of CLE lesions were treated with oral hydroxychloroquine, topical glucocorticosteroids, calcineurin inhibitors, and photoprotection. In addition, oral glucocorticosteroids and colchicine were also used in the treatment of CLE [6, 7, 10, 12, 15, 16, 19, 21]. For psoriatic lesions, topical glucocorticosteroids and methotrexate were the most common treatments [6, 7, 12, 13, 15–17, 19].
Due to the heterogeneous presentation of CLE, treatment must be individually adjusted for each patient. The basis of treatment includes protection against UV and sunlight exposure, cessation of smoking, careful analysis of prescribed medications, and avoidance of traumatic procedures [38]. Treatments for psoriasis encompass various modalities such as topical treatments, phototherapy, systemic medications, and targeted biological treatments. Topical glucocorticosteroids, calcipotriol, or their combination are the primary treatment for mild psoriasis. Oral therapies, including methotrexate, cyclosporine, and acitretin, are considered second-line options for moderate-to-severe psoriasis unresponsive to topical drugs [5, 39]. Based on the autoimmune pathogenesis of the psoriasis, targeted biological therapy has been introduced, encompassing TNF-α inhibitors (etanercept, infliximab, adalimumab), IL-17 inhibitors (secukinumab, ixekizumab), IL-12/IL-23p40 inhibitors (ustekinumab), IL-23 inhibitors (guselkumab, tildrakizumab, risankizumab, mirikizumab), IL-36/IL-1 inhibitors (spesolimab, imsidolimab), Janus kinase (JAK) inhibitors (tofacitinib, upadacitinib), and phosphodiesterase- 4 (PDE4) inhibitors (apremilast) [39].
CONCLUSIONS
This review provides a comprehensive overview of the literature regarding the coexistence of CLE and psoriasis, highlighting that, while this overlap is rare, SCLE and plaque psoriasis is the most commonly observed combination. The prevalence of this co-occurrence is notably higher in women. Treatment strategies generally follow established guidelines for each disease, though some therapies may be mutually exclusive or contraindicated. Clinicians must remain vigilant for the potential co-occurrence of these autoimmune diseases when evaluating and managing patients presenting with relevant symptoms, ensuring an individualized and effective therapeutic approach.