INTRODUCTION
Multiple sclerosis (MS) is a chronic and incurable disease of the central nervous system (CNS), with a complex etiology involving autoimmune, genetic, and environmental factors [1, 2]. An estimated 2.8 million people are currently living with MS worldwide and the prevalence of the disease is increasing. In Poland the number of patients with MS is about 50,000, with a prevalence of 120 per 100,000 people [3-5]. The vast majority of patients (66-91%) are initially diagnosed with relapsing-remitting MS (RRMS), which is characterized by the appearance of neurological symptoms accompanied by active, local inflammatory process in the CNS, alternating with periods of remission [2, 3, 5]. In most patients RRMS progresses to secondary progressive multiple sclerosis (SPMS), resulting in gradual neurological deterioration and permanent disability [6]. As MS mostly affects young adults, the socio-economic consequences of the disease are serious for both patients and countries [7]. Prevention of the development of disability is therefore crucial and, for this reason, proper treatment should be started as soon as possible. Therapy is most effective in the early years of the disease [2] and the costs of therapy are highly correlated with disease severity [8].
Prior to November 1, 2022, for patients with MS in Poland therapy began with first-line medicines (B.29 first-line drug program) [9]. If this treatment proved to be ineffective, patients might have been treated with highly effective second-line drugs (B.46 second-line drug program). However, the Polish drug program criteria for second-line therapy were very restrictive. To switch treatment, the patient must have had at least two moderate relapses and at least two new gadolinium-enhancing (Gd+) lesions or three new T2 lesions on magnetic resonance imaging (MRI) in the previous 12 months [10]. This made it impossible for patients without complete failure of first-line therapy to receive second-line therapy at the optimal time, despite their declining clinical status and MRI changes, leading to disease progression and increasing disability.
For this reason, in recent years only 8.5% of Polish patients with MS included in the therapeutic programs were estimated to be treated with second-line drugs [5]. The criteria of the Polish Neurological Society for second- line therapy are less restrictive. According to those criteria, highly effective therapy should be provided to patients after just one disease episode and one new lesion confirmed by MRI [2, 11].
The aim of the study was to evaluate the course of treatment in patients who, despite experiencing an active course of the disease, did not receive more efficacious treatment due to the criteria in the drug program in effect at the time. We analyzed the group of patients who did not meet the Polish drug program criteria for second-line treatment but who met the Polish Neurological Society criteria.
METHODS
Object of the study
The study involved neurologists working in treatment centers with an MS treatment program and involved adult patients with active RRMS who were or had previously been treated within the B.29 program.
Period of the study
Data were collected from September to November 2018. Physicians retrospectively described patients who had visited in the previous two months.
Data collection
The data were collected retrospectively by neurologists from patients’ medical documentation. Two data sources were used in the study: Form A (data about the number of patients with particular forms of MS) and Form B (data on demographics, course of disease, and treatment of patients with RRMS).
As this study was non-interventional and retrospective, ethical approval was not required.
All data collected during the study referred to routine medical management. No additional diagnostic or therapeutic interventions were conducted during the study, and no additional specialist consultations were performed.
Method of sampling
Two-stage sample selection was used.
• Stage 1: random-quota selection of physicians
Neurologists working in centers with a drug program for patients with MS were invited to participate in the study. They were selected to reflect the regional distribution of the number of this type of center in Poland and to ensure the representation of specialists from two types of center – those with one drug program (B.29), and those with two drug programs (B.29 and B.46).
First, treatment centers were randomly selected. In the event that the neurologists working in them refused to participate in the study, a procedure based on the geographical proximity of subsequent centers to those selected in the first step was applied.
• Stage 2: selection of patients
Each participating neurologist kept records of patients with MS who had visited in the previous 2 months (or less if there were more than 30 patients). They took into account the activity of the disease (i.e., the occur-rence of relapses and MRI changes throughout the whole treatment period, if the patient was receiving treatment). Patient records were collected in Form A.
Then, patients with RRMS who had active disease (at least one relapse of any severity and at least one new MRI lesion observed between the start of treatment in the drug program and the last visit) were selected. The relapse and MRI lesion did not have to occur simultaneously for the patient to meet the inclusion criteria. Inclusion of patients to the study was carried out in accordance with the order of their visit. Each doctor selected between 1 and 16 consecutive patients for the study according to the above-described scheme. The inclusion criterion for further analysis was current or previous treatment within the B.29 program for at least 12 months. Patients who had not previously been treated in the drug program were also eligible for the main study if they met a more severe criterion of disease activity (i.e. at least one new T1 lesion (Gd+) or two new T2 lesions [min. nine lesions in total] and at least two relapses with EDSS ≥ 1 in the previous 12 months). Patients who completed therapy within the B.46 program were excluded from the study. Data concerning patients’ medical history from the time of inclusion in the drug program until their last visit were then collected using Form B. Information was collected on treatment (drugs used, the duration of their use, and reasons for changing therapy) and disease activity (number and dates of relapses, with information about their severity, number, and dates of recorded T1 and T2 lesions in subsequent MRI scans).
In the next step, the above group of patients was limited only to those who do not meet the Polish drug program criteria for second-line treatment but who meet the Polish Neurological Society criteria (Table 1). Data for this population was subjected to detailed analysis.
Table 1
The Polish drug program criteria and the Polish Neurological Society criteria for second-line treatment (B.46 drug program) for patients treated with first-line treatment (B.29 drug program). To qualify for the second-line treatment, patients must meet both of these conditions
Analysis of first-line therapy
Medical records were used to assess the details of therapy, such as the type of current and entire treatment, the number and sequence of drugs administered, the duration of treatment, and reasons for discontinuing/ switching therapy.
Statistical analysis
Data were collected using online forms prepared in CADAS software. Statistical analyses were performed using the software package SPSS 20.0. Descriptive statistics were computed for analyzed parameters and the Shapiro- Wilk test was used to assess the distribution of variables. Differences between treatment duration were evaluated with the Mann-Whitney test.
RESULTS
Characteristics of the study population
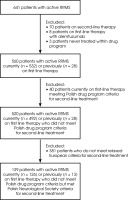
Criteria for active RRMS were met by 641 patients, of whom 139 (22%) currently or previously on first line therapy did not meet the Polish drug program inclusion criteria for second-line treatment but met the Polish Neurological Society inclusion criteria (Figure I). 126 patients were being treated in the B.29 program at the time and 13 patients had been treated previously (without treatment at the time of research due to pregnancy or discontinuation of medication).
The study population consisted of 89 (64%) women and 50 (36%) men. The median age was 37 years and ranged from 21 to 66 years. Detailed numbers of participants are shown in Table 2.
Table 2
The numbers of neurologists, treatment centers, and patients included in the main study
Treatment
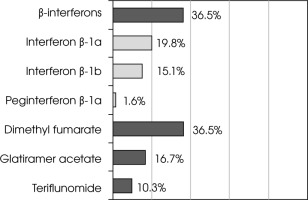
Among patients currently on treatment, β-interferons and dimethyl fumarate were equally commonly used. When evaluating medications individually, the most commonly applied drug was dimethyl fumarate (Figure II).
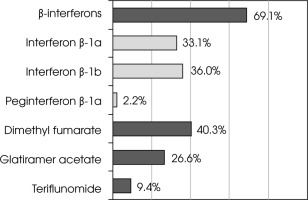
When analyzing the entire course of therapy to date, most patients were treated with β-interferons (interferon β-1b was most commonly used), followed by dimethyl fumarate. The least frequently used drug type was teriflunomide (Figure III).
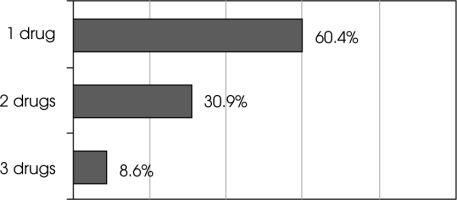
More than half of patients were treated with only one drug – mostly β-interferons, and despite clinical or MRI activity, treatment was not changed. However, almost 40% of patients underwent a change in treatment and two or three therapies were used (Figure IV).
In most cases, the first administered drug was one of β-interferons: in 36% of patients, it was the only drug used, and in 32% of patients who required drug switching, β-interferons were most commonly replaced by dimethyl fumarate. Initiation of treatment with glatiramer acetate occurred in 19% of patients, and initiation of treatment with dimethyl fumarate occurred in 12% of patients. When glatiramer acetate needed to be changed, dimethyl fumarate was applied in the vast majority of cases. In patients who started treatment with dimethyl fumarate, few drug changes were performed (Table 3).
Table 3
Sequence of medication usage (including current treatment) and median treatment duration with particular medications within each treatment pathway (in square brackets)
Treatment duration was assessed as the time from entry into the drug program to the last visit or completion of therapy, with interruptions in medication included. Currently administered therapy was also taken into account. The evaluated median treatment duration was 30.9 months.
The median duration of therapy with different drugs covered wide ranges, as follows: 6-59 months for glatiramer acetate, 3-63 months for β-interferons, 4-21 months for dimethyl fumarate, and 1-10 months for teriflunomide.
However, if the only treatment pathways considered were those with a frequency above 1%, the median treatment duration would be 30 months for β-interferons (n = 91), 27 months for glatiramer acetate (n = 35), 11 months for dimethyl fumarate (n = 52) and 4 months for teriflunomide (n = 7).
Tests were also performed to assess the differences between the duration of treatment with each drug used as first-line or second-line therapy. No significant differences were found between period of usage of interferon β-1a and interferon β-1b (p = 0.285); therefore, these drugs were analyzed together as β-interferons in further evaluations. No statistically significant differences in treatment duration were found between β-interferons and glatiramer acetate (p = 0.529). However, the duration of treatment differed significantly between dimethyl fumarate and β-interferons (p = 0.034) and between dimethyl fumarate and glatiramer acetate (p = 0.005).
Therapy modification with regard to the applied drug
Considering treatment discontinuation and switching as modifications of therapy, the percentage of treatment modifications was highest for interferon β-1a and lowest for teriflunomide (however, teriflunomide was used for a relatively short period) (Table 4).
Table 4
Percentage of modified and unmodified therapies depending on the applied drug
| Interferon β-1b | Interferon β-1a | Glatiramer acetate | Dimethyl fumarate | Peginterferon β-1a | Teriflunomide | |
|---|---|---|---|---|---|---|
| Modified therapies (switching or discontinuation) [%] | 97 | 100 | 76 | 26 | 66 | 0 |
| Unmodified therapies [%] | 3 | 0 | 24 | 74 | 34 | 100 |
The most common reason for discontinuation of first-line therapy was total or partial treatment ineffectiveness. Therapy was most rarely stopped due to maternity reasons and skin lesions (Table 5).
Table 5
Reasons for discontinuation of first-line treatment (cases where the drug was discontinued are shown; multi-answer question)
* Total failure was defined as lack of response to a full 12-month course of first-line treatment: 1) at least two moderate relapses (increase of 1-2 EDSS points) or one severe relapse after 6 months of treatment (increase of > 2 EDSS points) and 2) new lesions on MRI performed after month 12 (> 1 Gd+ lesion or > 2 T2 lesions); the two conditions must be met simultaneously. Partial failure was defined as the occurrence of only one of the above criteria.
The most common reason for drug switching was treatment failure, and the least frequently reported reason for drug switching was deterioration of blood parameters (Table 6).
Table 6
Reasons for drug switching during first-line treatment (cases where the drug was changed are shown; multi-answer question)
* Total failure was defined as lack of response to a full 12-month course of first-line treatment: 1) at least two moderate relapses (increase of 1-2 EDSS points) or one severe relapse after 6 months of treatment (increase of > 2 EDSS points) and 2) new lesions on MRI performed after month 12 (> 1 Gd+ lesion or > 2 T2 lesions); the two conditions must be met simultaneously. Partial failure was defined as the occurrence of only one of the above criteria.
DISCUSSION
Treatment of RRMS has advanced greatly since 1993, when the first disease-modifying therapy (DMT) – injectable interferon β-1b – was approved by the US Food and Drug Administration [12]. Nowadays, more than a dozen approved DMTs are available for MS, with varying mechanisms of action, routes of administration, efficacy, and side-effect profiles. Most of these target the active, inflammatory disease that defines RRMS [13]. β-interferons, as well as glatiramer acetate, teriflunomide, dimethyl fumarate, and fingolimod, were classified by the Association of British Neurologists as drugs of moderate efficacy (average relapse reduction in the 30-50% range). Drugs of high efficacy (average relapse reduction substantially more than 50%) are natalizumab (the first high-efficacy DMT) and alemtuzumab [14]. However, some authors of the network meta-analysis suggest that DMTs can be divided into three broad classes: drugs of low efficacy, including β-interferons, glatiramer acetate, and teriflunomide; drugs of moderate efficacy, including fingolimod, peginterferon β-1a, and dimethyl fumarate; and drugs of high efficacy, including alemtuzumab, ocrelizumab, and natalizumab [15]. According to another classification that also considered modern MS therapies, intramuscular interferon β-1a and teriflunomide 7 mg were classified as modest-efficacy drugs; dimethyl fumarate, glatiramer acetate, ozanimod 0.5 mg, and subcutaneous interferon β-1a and 1b were identified as moderate-efficacy therapy; fingolimod, ocrelizumab, and ozanimod 1.0 mg may be regarded and moderate- or high-efficacy drugs; and alemtuzumab, cladribine, ofatumumab, and natalizumab were classified as high-efficacy medications [16].
In Poland, drugs with low and moderate efficacy were used as first-line therapy and high-efficacy drugs were reserved as second-line treatments [9, 10]. In our study, conducted among patients treated with first-line therapy, the most commonly used medicines were β-interferons (especially interferon β-1b), which were applied with a frequency of 69% during the entire course of therapy. Our population was very specific because we included only patients with active RRMS on first-line treatment who did not meet the Polish drug program inclusion criteria for second-line treatment but who did meet the Polish Neurological Society inclusion criteria. Available publications mainly concern patients with RRMS in general, therefore direct comparison of our results with those of other studies might be influenced slightly by this difference. However, due to the lack of more relevant publications, our discussion will refer to available papers. Our observations seem to be comparable with the results of Polish multicenter studies published in 2010 and in 2020, in which β-interferons were administered to 81% and 63% of patients, respectively [17, 18], and also to the results of studies from other countries, in which β-interferons were the most commonly administered drugs [19, 20]. The second most frequently (40%) administered medication during the entire course of therapy in our study was dimethyl fumarate, which is classified as having slightly higher efficacy than β-interferons [15].
In most patients, treatment began with the administration of β-interferons and more than half of patients were treated with only one drug. In most cases, this was a β-interferon. This is most likely due to the fact that β-interferons have been available for the longest time in Poland (since 2004) compared to other drugs. This also confirms the safety of long-term use of β-interferons.
Patients who needed to change therapy were usually switched to dimethyl fumarate. Only two of 17 patients who started therapy with dimethyl fumarate had their treatment changed. This might be due to the fact that dimethyl fumarate is thought to have better efficacy than β-interferons; however, this observation might be influenced significantly by the route of drug administration as dimethyl fumarate is administered per os, and better compliance and persistence with oral than injectable therapies has been shown [21, 22]. This assumption seems to be supported by the fact that, among 39 patients who had a previous drug switched to dimethyl fumarate, as many as 36 remained on dimethyl fumarate. Besides, during the study period dimethyl fumarate had been available in the drug program for a relatively short period (since 2016), which could affect the results.
However, this supposition was not reflected in the duration of therapy, with dimethyl fumarate compared to injectable drugs, which could be caused by the fact that in five of the eighteen noted treatment pathways dimethyl fumarate is the currently administered drug, and therefore the actual duration of use of this drug may become longer. Initiation of therapy with oral medication in our study was noted in only 12% of cases (only dimethyl fumarate); however, in an Italian population of patients with RRMS this percentage was as high as 50% [23]. This may be explained by the availability of the drug. Dimethyl fumarate has been used in many countries in Europe since January 2014, whereas Polish patients have been receiving it since July 2016. However, its use is steadily increasing, and it has been the drug with which treatment was most often started among Polish patients in recent years [18, 24]. This indicates that neurologists are convinced of the need to start treatment with the most effective treatments available.
Regarding oral DMTs, Vermersh et al. observed that persistence was higher on teriflunomide than on dimethyl fumarate [21]. We noted the same in our study – no treatment modification with this drug was made. However, the duration of therapy with teriflunomide in our study was relatively short (median of 1-10 months), probably due to it being available for a shorter time (since 2017).
The main cause of therapy discontinuation was treatment ineffectiveness (total or partial). Similar observations were made in a Polish multicenter study [18]. This was also the main cause of switching to another drug. In publications by other authors, lack of treatment effectiveness was also the main reason for treatment modification [24-26]. In our study one third of patients switched only once and almost 10% of patients had three drugs administered. The drugs most frequently switched to another drug were β-interferons. In most cases, the switch was made to dimethyl fumarate.
With the number of all-treatment modifications (discontinuation and switching), it was noted that their number was the highest in case of interferon β-1a and interferon β-1b therapies. In turn, the lowest percentage of treatment modifications was observed during therapy with teriflunomide and dimethyl fumarate. These observation are supported by real-world data from the Italian register that suggests that first-line oral DMTs are associated with a lower risk of therapy discontinuation compared to injectable DMTs [27]. Our results seem to be in accordance with outcomes from a network meta-analysis that showed that interferon β-1a, interferon β-1b, and glatiramer acetate were associated with generally inferior acceptability profiles compared with the other DMTs [15].
MS treatment is a very dynamic process in which the course and activity of the disease, as well as the patient’s characteristics and preferences, are taken into account. However, reimbursement and availability of therapies is also a key element. Considering therapeutic programs in Poland, the introduction of new, more active therapies results in their use in a wide range of patients.
Our study showed that nearly a quarter of patients with RRMS who received first-line DMTs would be eligible for second-line treatment, regardless of disease activity. However, prior to November 1, 2022, only changes to other first-line DMTs were possible, and patients were receiving suboptimal treatment, often for many years. This led to increasing brain damage and worse prognoses.
According to current expert opinion, early administration of high-efficacy DMTs might represent the best approach to delaying irreversible CNS damage and the progression of disability. Therefore, early use of high-efficacy medication should be mandatory in the treatment of people with MS in whom prognostic factors suggest aggressive disease. The opportunity to apply high-efficacy drugs early after diagnosis would be highly desirable [28].
Our data indicate that highly active therapies could have been used earlier in 22% of RRMS patients treated in the drug program in Poland and suggest the need for faster and wider access to such treatment. The new drug program introduced since November 1, 2022 enables early therapy with some highly effective drugs, which may improve treatment efficacy and prognosis.
CONCLUSIONS
First-line therapy of patients with active RRMS was based mainly on β-interferons and dimethyl fumarate. For most medications, discontinuation of therapy or drug switching were very common and the main reason was treatment failure.
However, due to the restrictive criteria of the drug program, patients did not receive more effective treatment at that time, and some of them required subsequent changes in therapy due to persistent disease activity.
These observations suggest the need for faster and wider access to treatment with highly effective drugs in the studied population.