INTRODUCTION
Cancer is the main cause of death in people younger than 85 years old. Among men and women, colorectal cancer was the fourth-leading cause of death in the late 1990s. However, it is now the first in men and the second in women [1]. More than a quarter of all cancers are gastrointestinal (GI) cancers, which include stomach, liver, esophageal, pancreatic, and colorectal cancers; additionally, their prevalence is continuously growing [2]. Therefore, it is important to know the correlation between skin manifestations and potential gastrointestinal disease due to the fact that the cutaneous and GI systems are linked in origin, and awareness of these skin manifestations might have an important diagnostic value in detecting malignancies earlier with possible life-saving outcomes. Moreover, a great number of cutaneous manifestations herald the clinical symptoms of an underlying GI disease [3, 4]. In some cases, cancer cells found in the skin may represent metastasis from a GI malignancy; in others, skin signs do not have cancer cells and can be regarded as paraneoplastic dermatological syndromes [5, 6]. The aim of this review is to concisely summarize both well-established and recent literature regarding skin manifestations associated with GI tract cancers, thereby supporting vigilance in daily clinical practice. In this paper, the terms “cancers” and “malignant neoplasms” are used interchangeably to refer to malignant tumors of the gastrointestinal tract.
PARANEOPLASTIC SKIN MANIFESTATIONS
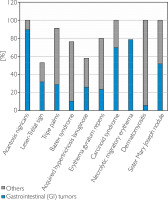
Paraneoplastic syndromes are a group of rare disorders associated with existing malignancy, without direct involvement of tumor invasion or metastasis. These syndromes arise indirectly through factors produced by the neoplastic process such as hormones, cytokines, polypeptides, or antibodies, which disrupt normal cellular com- munication and function. Paraneoplastic syndromes can affect multiple organ systems and may manifest both clinically and biochemically. Figure 1 illustrates the association between different paraneoplastic syndromes and cancer incidence, stratified by tumor origin.
Figure 1
Association of paraneoplastic syndromes (x-axis) with cancer incidence (y-axis), stratified by tumor origin. Data are based on published literature [31, 77–83]
Paraneoplastic skin manifestations are a group of skin disorders characterized by variable clinical presentation and often benign appearance. Establishing the correlation between the dermatological condition and ongoing malignancy can be challenging as it might manifest in parallel with the malignancy, precede it, or appear late, after the neoplastic process has already started.
In this narrative review, the authors concentrated on analyzing the involvement of the skin in paraneoplastic syndromes, including them in the following order: acanthosis nigricans, Leser-Trélat sign, tripe palms, Bazex syndrome, acquired hypertrichosis lanuginosa, erythema gyratum repens, carcinoid syndrome, necrolytic migratory erythema, dermatomyositis, and Sister Mary Joseph nodule, which, although discussed in this review, is an example of direct metastasis of the cancer [7].
ACANTHOSIS NIGRICANS
Acanthosis nigricans (AN) is a relatively common example of paraneoplastic dermatosis [7, 8]. However, internal malignancies are rarely identified as primary etiological contributors to this cutaneous condition [9]. AN may present in benign or malignant forms [7]. The benign form can be familial or associated with obesity, systemic glucocorticoid use, oral contraceptives, or as a marker of hyperinsulinemia and insulin resistance (IR) [7–9].
However, Kumar et al. described a patient with AN as a consequence of hepatitis C virus (HCV) infection and IR. The proposed mechanism suggests that hyperinsulinemia, as a consequence of HCV, leads to increased levels of insulin-like growth factor 1 (IGF-1), which acts as a fibroblast stimulator, resulting in AN [10].
Benign AN may present itself as hyperpigmented plaques in the areas of the neck (“dirty neck” look), intertriginous (axillae, inframammary folds, inguinal), and popliteal fossae, antecubital [7]. Sometimes, the diagnosis of AN might be confusing. Alshareef et al. demonstrated the case of a 5-year-old boy with type 1 diabetes mellitus (DM), in whom AN manifested as multiple tiny, non-scaly brownish papules located bilaterally on the medial parts of the upper thighs [11]. The malignant AN usually appears in adults with an average age of 40. Acanthosis nigricans maligna (ANM) is more extensive and severe than the benign form. It manifests itself as a sudden onset of symmetrical hyperpigmentations that occur in atypical areas (such as knuckles, palms, eyelids, perioral, and interdigital space), although any skin area can be involved.
Later, the lesions may develop into velvety plaques, commonly surrounded by acrochordons. The mucosal area, anal, oral, and genital mucosa might be involved with flesh-colored papules; however, this has been rarely described [7, 8].
Interestingly, Liu et al. provided the case of a 30-year-old woman who developed papillomatosis with hyperemia on the lips, hard palate, and gingiva, along with velvety hyperplasia on the inner cheeks and tongue. These symptoms, due to their similarity, were initially misdiagnosed as a seafood allergy [12]. The authors summarized data on AN with oral manifestations associated with malignancy. Papillomatosis was the most common manifestation, occurring in 72.2% of cases; nearly 60% of patients developed oral lesions before their cancer diagnosis. Therefore, recognizing these symptoms can offer valuable diagnostic clues and may, in some cases, be life-saving [12, 13].
Noteworthy is that clinically, the differentiation between benign and malignant acanthosis nigricans is not possible [9]. The warning signs of potential malignancy include the rapid onset of AN and the presence of other paraneoplastic syndromes, such as multiple seborrheic keratoses (SK). Up to 25% of patients with ANM might present with tripe palms (TP), therefore, it is crucial to follow up with the patients in order to detect a possible underlying malignancy [8, 9].
LESER-TRÉLAT SIGN
Leser-Trélat sign (LTS) is a rare paraneoplastic syndrome presenting as a sudden occurrence of multiple seborrheic keratoses, primarily affecting the thorax and dorsum, in patients with internal malignancies [14, 15]. It mostly occurs in gastrointestinal adenocarcinomas, as well as in lung, breast, and pancreatic cancers [16].
Cases of LTS have also been reported in non-malignant conditions, including human papillomavirus (HPV) or human immunodeficiency virus (HIV) infection, during pregnancy, or following cytarabine therapy [17, 18]. In addition, LTS resembling that observed in adenocarcinomas has been reported secondary to in situ melanoma [19]. Furthermore, LTS occurring approximately 2 months after COVID-19 recovery has been documented [20]. Exclusion of underlying malignancy is essential, as these conditions may mimic LTS.
The patients with LTS have an average age of onset of 61 years. There are no reports of an increased predilection amongst either sex or race [16]. The pathophysiology of its development is difficult to define, yet some experts suspect that growth factors and cytokines that are released from malignant neoplasm contribute to the eruptive growth of seborrheic keratosis. In addition, there are no quantified or standardized criteria currently available to diagnose the Leser-Trélat sign [16, 18]. In some cases, LTS might be mistaken for other conditions, such as pemphigus; therefore, antibody testing should be performed when there is any uncertainty [21]. Distinguishing LTS from benign seborrheic keratoses can be challenging as their benign form is common, mostly harmless, and often appears gradually, especially in individuals after 80 [22]. Additionally, it is important to differentiate LTS from pseudo-LTS, where, as mentioned, no malignancy is present [18, 23]. As in other paraneoplastic syndromes, pruritus, referred to as paraneoplastic itch (PI), might be present, although it is considered more of a reaction to the presence of a cancer. Interestingly, Werda et al. emphasized the effectiveness of SSRIs (selective serotonin reuptake inhibitors), instead of antihistamines [24].
TRIPE PALMS
Tripe palms (TP) is an unusual skin disorder characterized by rugose thickening of the palm that has been referred to as hyperkeratosis palmaris, acanthosis palmaris nigra, acanthosis palmaris, and keratoma palmaris [25, 26]. It was first introduced in the literature in 1977 by Jacqueline Clarke in a patient with squamous cell carcinoma (SCC) of the lung [25]. It can be disclosed alone; however, it might also be found in connection with AN, with more than 50% of cases associated with gastric and lung cancers [26].
The coexistence of ANM with TP was described in a young woman diagnosed with primary mediastinal B-cell lymphoma, which, in contrast to Hodgkin lymphoma, is not a common cause of these skin manifestations. Interestingly, the accompanying itch is attributed not to the altered skin structure but to the irritation of nerve endings by cytokines released in response to the lymphoma cells [27]. Additionally, TP has been reported in association with COVID-19 infection, along with AN. In that case, a lung transplant in the patient caused the regression of skin lesions [28].
In the differentiation of TP, it is important to consider palmoplantar keratoderma, which might be present in PTEN-positive (Phosphatase and Tensin homolog) Cowden syndrome, and also occurs on the palms and soles. However, in contrast to TP, the skin texture in palmoplantar keratoderma is rough [29].
BAZEX SYNDROME
Acrokeratosis paraneoplastica, commonly known as ‘Bazex syndrome’ (BS), is a relatively rare condition associated with squamous cell carcinoma (SCC) of the upper GI and respiratory tracts, including SCC of the lung. It may also precede malignancies such as breast or cervical cancer [30]. Smoking cigarettes and alcohol intake are risk factors for BS [31]. Acrokeratosis paraneoplastica affects mostly men over 40 and tends to occur on the acral parts of the body, such as the ears, nose, soles, or palms, accompanied by nail alterations. Horton et al. noted that while skin lesions typically appear about 1 year before cancer, approximately 20% of cases exhibit BS lesions in parallel with a malignant neoplasm [32]. Furthermore, Shah et al. emphasized that, although skin manifestations appear at least six months before cancer, in 30% of cases the situation is reversed, with skin changes manifesting after the cancer is diagnosed [30].
The clinical features typically present as symmetrical, psoriasis-like cutaneous plaques with scaling. Violaceous erythema, erosions, yellowish crusts, or seldom blistering lesions may be observed [31]. Nail involvement, often the first sign, is characterized by erythema with swelling of the perionychium, ridging, yellow discoloration, onycholysis, nail dystrophy, and subungual hyperkeratosis [32, 33]. The suggested mechanism of the formation of skin lesions assumes the stimulation of keratinocytes by growth factors secreted by SCC [34].
BS develops in three levels of progression. The first encompasses skin lesions symmetrically on the ears and nose. The second stage extends to the palms and soles, with hyperkeratosis, fissures, and nail destruction. The third stage is considered a reflection of cancer metastasis or therapy failure, spreads to the trunk and dorsal parts of the feet and hands [31]. As reported in the case of a patient with stage IV cancer of the base of the tongue, with no response to chemotherapy, it was described as the third, generalized stage of acrokeratosis paraneoplastica [33]. It is believed that in most cases, curing the cancer is the best treatment for BS. Conversely, the recurrence of cancer might lead to the recurrence of skin lesions as well, making them a good indicator of cancer’s progression [32, 33]. According to Pulickal and Kaliyadan, there are some observations suggesting the efficiency of both systemic and oral retinoids in the alleviation of skin lesions. The features of BS can resemble psoriasis or eczema, and, as a result, it is often misdiagnosed and ineffectively treated. What usually differentiates these conditions is their location; however, the nail changes are indistinguishable from psoriatic involvement [30, 31].
ACQUIRED HYPERTRICHOSIS LANUGINOSA
Hypertrichosis means excessive hair growth that may occur in both sexes and on any area of the body. Unlike hirsutism, it is not related to androgen-dependent regions [35]. Acquired hypertrichosis lanuginosa (AHL) is a paraneoplastic disorder that typically presents with lung and colorectal cancer. Less commonly, it is associated with breast cancer or leukemia. It is characterized by lanugo-type hair, notable for its fineness and lack of pigment in the shafts. The face is a particularly prevalent location for hypertrichosis, but it can also affect the trunk or extremities [35, 36]. In some cases, acquired hypertrichosis may be accompanied by trichomegaly of the eyelashes and eyebrows [36]. Interestingly, Revés et al. described the case where, in addition to hair growth, the patient also reported a painful, red, and fissured tongue [37]. Yim et al. presented the case report where AHL was diagnosed in a woman suffering from autoimmune hepatitis. Apart from this rare case, the authors emphasized that besides malignant neoplasms, there are other causes of AHL, which might include anorexia nervosa or some medications, including cyclosporine. Given the absence of these conditions in the patient’s medical history, the possibility of an underlying malignancy should be strongly considered [38].
ERYTHEMA GYRATUM REPENS
Erythema gyratum repens (EGR) is an uncommon dermatological disorder, often regarded as a paraneoplastic condition, occurring twice as frequently in men. It typically appears a few months before a malignant neoplasm is diagnosed, but the possibility of its developing alongside cancer or even several years later cannot be completely excluded. The most common associated cancers are lung cancer, followed by esophageal and breast cancer [39, 40]. There have also been reported cases of benign conditions, accompanied by EGR, such as Raynaud’s phenomenon, tuberculosis, or in postpartum women [40, 41]. Recently, Elhage et al. presented the case of EGR as a secondary manifestation of COVID-19. The lesions appeared 2 weeks after a positive RT-PCR test and lasted for 10 days. In comparison, another case report described EGR appearing 30 days after the COVID-19 diagnosis and lasting for 60 days [42].
The clinical presentation of erythema gyratum repens (EGR) is characterized by erythematous, scaling rings arranged in a serpiginous pattern, resembling wood-grain rings [42, 43]. It might be located on any surface of the body, except palmar and plantar [40]. Sometimes, due to its atopic-like appearance, it is mistakenly treated with immunosuppressants [44, 45]. The treatment of EGR is mainly based on curing the underlying disease [41]. Dominiak et al. reported a case of EGR mimicry in a woman presenting with bullae arranged in a wood-grain pattern. However, both skin and serum immunofluorescence results indicated bullous pemphigoid [46]. Another case report, highlighting the necessity of further diagnostics, was presented by Campbell et al. They presented a male patient with a 10-month history of pruritic, EGR-like lesions, initially misdiagnosed and treated as psoriasis. Malignancy was subsequently excluded; of note, his family history was positive for maternal lung and brain cancers [47].
CARCINOID SYNDROME
Carcinoid is a type of tumor that originates from the endodermal germ layer, developing from enterochromaffin cells, with neuroendocrine properties. Due to their secretory properties, they are a subset of neuroendocrine tumors (NETs). The most commonly affected system is the GI tract, with the prevalence of the small intestine. Bronchial carcinoids account for a small percentage of cases [48]. Deme et al. reported that none of the 62 patients with bronchopulmonary neuroendocrine tumors (BPNETs) included in their study exhibited symptoms of carcinoid syndrome [49]. Chemical compounds secreted by the tumor cells may lead to a range of symptoms, including skin flushing, diarrhea, and right heart dysfunction, due to the formation of carcinoid plaques on the valves [50, 51]. These manifestations constitute carcinoid syndrome (CS), with flushing present in the majority of patients. CS is estimated to occur in approximately 10% of carcinoid tumors [52]. The presence of skin flushing is strongly associated with liver metastases [53]. Certain mediators, such as substance P and histamine, secreted by hepatocytes distal to the portal vein, contribute to flushing [52]. Nevertheless, according to the Surveillance, Epidemiology, and End Results (SEER) database, carcinoid syndrome can also be observed in patients with localized or regional disease [54]. The location and triggering factors are very similar in carcinoid syndrome and physiological events; thus, the flushing typically occurs in the head, neck, and upper trunk, and can be triggered by amine-rich food or anger [52]. Additionally, the type of flushing might indicate a specific tumor location: midgut tumors tend to cause a brief cyanotic flush with a burning sensation, while foregut tumors can cause pruritic, reddish-brown wheals that may appear on the entire body. Other manifestations, such as diarrhea or abdominal pain, are common and help distinguish carcinoid syndrome from other flushingrelated diseases [55].
Besides flushing, the rare skin manifestations of CS can include scleroderma-like lesions. Koch and Grayson described the case of a woman with CS, with a tumor primarily arising from the intestine. She complained of erythema and stiffness in her lower legs. No autoantibodies were detected, indicating that autoimmunity was not the underlying cause [56].
Sometimes, CS might be mimicked by other conditions. Latta et al. presented a man whose significant weight loss, watery stools, and skin flushing led to a suspicion of CS. However, Strongyloides infection was detected, and none of the larva currens symptoms or hives were observed [57]. This case highlights the diagnostic challenges and the necessity for differentiation from other conditions that cause rosacealike flushing, such as mastocytosis, pheochromocytoma, or endogenous Cushing’s syndrome [58].
NECROLYTIC MIGRATORY ERYTHEMA
Glucagonoma is a rare and usually malignant neoplasm of the pancreatic islet α-cells that can induce numerous manifestations such as diabetes mellitus, anemia, weight loss, glossitis, cheilitis, necrolytic migratory erythema (NME), and neuropsychiatric disturbances [59, 60]. Owing to its rarity, the diagnosis is often delayed [59, 61]. It is important to remember that the vast numbers of cases of NME are associated with pancreatic neuroendocrine tumors (PNETs). NME is a superficial epidermal necrosis with central bullae or crusts, most commonly affecting the perineum, lower abdomen, face, and distal extremities [60, 62]. It is considered a key feature in the diagnosis of glucagonoma syndrome, occurring in approximately 70% of glucagonoma syndrome (GS) [59]. Nevertheless, an atypical case with scaly pustules located in the periauricular region was also described [63]. Moreover, morbidity from PNETs is mostly because of malnutrition and NME, which can be partially improved by the usage of amino acid infusions, antibiotics, and somatostatin [61]. However, difficulties in GS diagnosis are possible. Abdelli et al. described the intriguing case of a woman with polymorphous skin lesions, which were misdiagnosed several times and included eczema-like eruptions or insect bite eruptions. Thus, mimicking clinical features might delay the diagnosis, especially when the primary feature of GS appears without other typical symptoms indicating GS [64].
Establishing a differential diagnosis for glucagonoma syndrome can be particularly complex due to its variable clinical presentation. Juśko et al. described a patient in whom necrolytic migratory erythema (NME) was initially misdiagnosed as drug-induced erythema multiforme. Initial imaging studies revealed no abnormalities. As the cutaneous lesions progressed, alternative diagnoses such as psoriasis, pityriasis rubra pilaris, eczema, and allergic contact dermatitis were considered. Subsequent radiologic evaluations ultimately identified a pancreatic neuroendocrine tumor (PNET) [65].
DERMATOMYOSITIS
Dermatomyositis (DM) is the most common (~30– 40%) subtype of idiopathic inflammatory myopathies (IIM) [66]. There are a few known risk factors that include, for instance, genetics, ultraviolet radiation, female sex, and preceding respiratory diseases [67]. It is a systemic autoimmune disease associated with a predominant proximal muscle weakness along with skin symptoms, in particular heliotrope erythema and Gottron sign or papules [66–68]. Due to muscle weakness, the patient can experience difficulties with running, climbing stairs, and lifting their arms [66, 68]. While the causes of most IIM cases remain unknown, some occurrences are reported as consequences of paraneoplastic syndromes [66]. In adults, 20–50% of dermatomyositis (DM) cases are associated with an underlying malignancy, whereas this correlation is not observed in children [69]. While transcription intermediary factor 1 (TIF-1) antibodies are associated with malignancies in approximately 75% of adult cases, they are not considered a marker of cancer in the pediatric population indicating juvenile dermatomyositis instead (JDM). Like many other paraneoplastic syndromes, they are not a direct consequence of malignant neoplasm, but rather due to an immune response to malignancy [68].
According to the retrospective study by Chang et al., the most common types of cancer associated with DM were lung and esophageal cancer in men, whereas in women, thyroid, breast, and cervical cancers were predominant. Around 68% of examined patients were diagnosed with cancer after their initial diagnosis of DM, mostly within 1 year [70].
Due to facial skin changes presenting as erythema with scaling and accompanying pruritus, common conditions such as contact and photocontact dermatitis, rosacea, seborrheic dermatitis, and psoriasis should be considered. Muscle pain, however, necessitates differentiation from disorders such as SCLE and SLE [71].
SISTER MARY JOSEPH NODULE
Sister Mary Joseph nodule (SMJN), although mentioned in this review, should not be considered a paraneoplastic syndrome. It represents a direct cutaneous metastasis from an underlying malignancy. Clinically, SMJN presents as a firm, ulcerated, and often painful mass, frequently accompanied by bloody or purulent discharge. The size of SMJ is usually about 5 mm; however, larger ones have been described in the literature. The color of the nodule can be bluish-violet, white, or brownish-red. This umbilical skin metastasis represents a rare spread from intra-abdominal cancers of gastrointestinal or genitourinary origin [72, 73]. Cancers of the colon and stomach are commonly associated with SMJ, while the pancreas is a less common primary site. If pancreatic adenocarcinoma metastasizes to the umbilicus, it typically arises from the tail or the body of the pancreas, as demonstrated in the case of a patient presented by Leyrat et al. In this case report, three periumbilical nodules appeared during second-line chemotherapy treatment, which correlated with disease progression as revealed on a computed tomography (CT) scan [74, 75].
Without treatment of cancer, the average survival time is approximately 2 months [73]. Moreover, it is associated with a poor prognosis and implies advanced cancer [72–75].
SMJ can sometimes be misdiagnosed as an umbilical hernia, a common condition, and may not be immediately recognized as umbilical metastasis even after imaging diagnostics. Conditions worth differentiating include endometriosis, sarcoma, lymphangioma, lipoma, and granuloma [76].
CONCLUSIONS
Skin manifestations of gastrointestinal disorders, although they can be quite rare, may provide significant clues in diagnosing cancer. Many physicians, particularly gastroenterologists, internists, and general practitioners, should be familiar with possible skin symptoms resulting from ongoing pathologies within a patient’s body. As noted, these manifestations often precede organ disease, which can significantly impact the patient’s clinical trajectory. However, many of these manifestations are non-specific, which may lead to the initiation of incorrect treatment. Patients presenting with any skin manifestations, in combination with symptoms from the digestive tract, should be carefully screened by a dermatologist. If malignancy is suspected, further diagnostic evaluations should be performed, including routine medical assessments such as ultrasound or endoscopic examinations of the gastrointestinal tract.








