INTRODUCTION
Nevus sebaceous (NS) is a congenital skin malformation usually located on the scalp, forehead, and neck, with the potential for both benign and malignant tumor development [1]. NS progresses through stages, with an initial enlargement and darkening during childhood, followed by an increasing risk of malignant transformation in adulthood, including basal cell carcinoma (BCC) and squamous cell carcinoma (SCC) [1]. However, since the overall risk of malignancy is considered low, the prophylactic removal of all NS lesions remains debatable [2]. This systematic review aimed to assess the prevalence, age, and sex distributions of NS-related tumors, providing insights into the characteristics and likelihood of tumor development. These findings have important implications for reevaluating current practices in the excision of NS, which are still frequently performed.
METHODS
This study followed the Cochrane Collaboration and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and Meta-analysis of Observational Studies in Epidemiology (MOOSE) reporting guidelines, and it was registered in the international Prospective Register of Systematic Reviews (PROSPERO) under protocol number CRD42024533102.
The inclusion criteria were as follows: (1) trials, observational studies, and case series with more than 10 cases; (2) enrolling patients with NS; (3) published after 1993; and (4) reporting at least one outcome of interest. Exclusion criteria were: (1) studies with overlapping populations; (2) non-original studies; (3) gray literature; and (4) case reports. No language or geographic restrictions were applied.
To minimize the misdiagnosis of trichoblastoma as basal cell carcinoma (BCC) in studies dated before 1993, we included only investigations occurring thereafter.
The Embase, PubMed, and Cochrane databases were sought from 1993 to March 2024 with the following search terms: “secondary tumors”, “tumors”, “neoplasms”, “tumors”, “neoplastic lesions”, “trichoblastoma”, “malignancies”, “carcinoma”, “basal cell carcinoma”, “sebaceous nevus”, “nevus sebaceous”, “nevus sebaceus”, “organoid nevus”, “nevus sebaceus of Jadassohn”, “Jadassohn’s nevus”, “Jadassohn’s Nevus Sebaceous”, “naevus sebaceous”, and “nevus sebaceous”. Backward searches were conducted to identify additional relevant articles. Two authors (F.L. and M.M.B.) independently reviewed the studies. Disagreements were addressed through consensus.
The outcomes of interest were: (1) the overall proportion of NS-related tumors; (2) the rate of both benign and malignant tumors; (3) the types of benign and malignant NS-related tumors; and (4) the frequency of patients with more than one neoplasm secondary to a single NS.
The presence of two or more secondary tumors within a single lesion was considered a separate diagnosis for statistical analysis. Multiple biopsy samples or consecutive excisions from a single lesion were considered as only one diagnosis. Benign lesions included, but were not limited to, warts, cysts, and nevi. Keratoacanthoma was categorized as a malignant growth. Subgroup analyses based on gender and age were performed for both benign and malignant NS-related tumors.
The Newcastle-Ottawa Scale (NOS) was used to assess the risk of bias of the included studies. Two authors (B.X.M. and Y.S.S.) assessed the quality of the included studies. Disagreements were resolved by consensus.
RESULTS
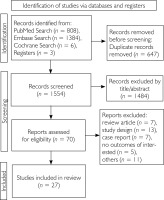
The database systematic search yielded 2,198 records. Following the removal of duplicates on Zotero, the titles and abstracts of the remaining studies were screened in Rayyan, leading to the identification of there is no 67 in the flow diagram articles for full review as shown in the flow diagram of study screening and selection (fig. 1). A total of 27 observational studies meeting the eligibility criteria were included, covering a total of 7,092 NS. The sample size varied from 13 to 729 across the studies. Geographically, there were eleven studies conducted in Asia, eight in Europe, seven in North America, and one in Central America. The detailed study characteristics are listed in table 1.
Table 1
The main findings of the included studies of included studies
| Study, country | Total NS (n) | Female (%) | Age [years] | NS located on the scalp | Secondary lesions (n) | Benign lesions (n) | Malignant tumors (n) |
|---|---|---|---|---|---|---|---|
| Barba 2009 [10] Mexico | 260 | 55 | 29.4 | 61.5 | 33 | TB (16); SCAP (6); TM (2); PE (2); AD (2); comedonal nevus (1); nodular hidradenoma (1) | BCC (1) |
| Barkham 2007 [2], USA | 63 | NA | 6.7 | NA | 1 | AD (1) | 0 |
| Beer 1999 [11], Austria | 18 | NA | 24.0 | NA | 6 | SCAP (1) | BCC (4): keratoacanthoma (1) |
| Burli 2022 [12], USA | 73 | NA | 10.2 | 68.5 | 6 | Verrucous papule (6) | 0 |
| Chundriger 2020 [13]; Pakistan | 111 | 40.5 | 31.2 | 45.9 | 23 | SCAP (9); TE (2); Epidermal nevus (4); others (6)** | BCC (2) |
| Chun 1995 [14]; Puerto Rico | 165 | 58.8 | NA | NA | 9 | SCAP (3); TB (5); AD (1) | 0 |
| Cribier 2000 [15]; France | 596 | 48.7 | 25.4 Range: 1–8 | 49.8 | 118 | SCAP (30); TB (28); TM (16); sebaceoma (13); epidermal nevus (5); SK (3); VW (14) | BCC (5); KA (4) |
| Davisson 2004 [6], USA | 13 | NA | NA | 100 | 2 | 0 | BCC (2) |
| Gao 2019 [17], China | 251 | 43.4 | 25.8 Range: 5–77 | 61.8 | 34 | SCAP (9); TB (7); TM (3); VW (3); sebaceoma (2); MN (1); syringoma (1) | BCC (4); keratoacanthoma (2); SC (2) |
| Goel 2019 [18], USA | 92 | 41.3 | 7.24 Range: 0.5–16 | 100 | NA | NA** | 0 |
| Harsan 2017 [19], Vietnam | 59 | NA | NA | NA | 22 | TB (7); syringoma (4); sebaceoma (1); SK (2); basal cell hamartoma (1) | BCC (4); SCC (2); keratoacanthoma (1) |
| Hsu 2016 [20], Taiwan | 450 | 47.3 | 23.7 | 61.7 | 38 | SCAP (12); TB (7); TM (7); sebaceoma (3); AD (1); IFK (1) | BCC (4); SCC (2); SC (1) |
| Idriss 2014 [21], USA | 707 | 43.3 | 27.4 | 62.5 | 159 | TB (52); SCAP (33): A/E D (15); MN (7); TFI (7); VW (5); TM (8); Cyst (2) sebaceoma (2); AK (1); adenomyoepithelioma (1) | BCC (8); SCC (4); SC (3); AC (1); MAC (1) |
| Jaqueti 2000 [22], Spain | 155 | NA | NA | 59.3 | 56 | Any warts (18); TB (12); SCAP (10); sebomatricoma (8); TM (4); apocrine hidrocystoma (3); poroma (1) | 0 |
| Kamyab-Hesari 2016 [23], Iran | 168 | 51.2 | 21.1 Range: 2–62 | 57.1 | 9 | TB (4); TM (3); SCAP (2) | 0 |
| Lee 2020 [6], South Korea | 531 | NA | NA | NA | 29 | TB (4); SCAP (4)** | BCC (6); SCC (4) |
| Lytvynenko 2017 [24], Ukraine | 153 | 37.4 | 36.4 | 76.5 | 37 | Total (20)** | BCC (13); SCC (4) |
| Minami 1998 [25], Japan | 136 | 50.7 | NA | NA | 18 | SCAP (4); TB (3); sebaceoma (3)** | BCC (4); SC (3) |
| Muñoz-Pérez 2002 [26], Spain | 226 | 48.6 | NA | 93 | 49 | SCAP (29); TB (10); AD (2) | BCC (8) |
| Rosen 2009 [27], USA | 651 | 47.9 | 7.2 Range: 0.3–54.3 | 62.8 | 21 | SCAP (7); MN (4); TM (1); AD (1); TE (1); combined nevus (1); syringoma (1) | BCC (5) |
| Santibanez-Gallerani 2003 [9], USA | 658 | 49.0 | Range: 2–16 | NA | NA | NA | 0 |
| Serrano 2003 [28], Spain | 366 | 41.0 | 38.0 | 65.3 | 51 | SCAP (12); TB (8); VW (15); sebaceoma (4); poroma (3); sebaceous hyperplasia (1) | BCC (7); VC (1) |
| Simi 2008 [29], India | 21 | 52.4 | Range: 8–68 | 57.1 | 3 | Cyst (2) | SCC (1) |
| Takizawa 2002 [30], Japan | 46 | NA | 23.9 | NA | 5 | SCAP (1); TM (1) | BCC (3) |
| Van 2019 [31], Vietnam | 38 | NA | NA | 73.7 | 3 | TM (1); Hidradenoma papilliferum (1) | BCC (1) |
| Wang 2020 [32], China | 729 | 40.5 | 16.16 | NA | 56 | SCAP (12); TB (9); MN (2); sebaceoma (1); lipomatous nevus (1)** | BCC (5) |
| Ye 2023 [4], China | 356 | NA | NA | NA | 54 | TB (16); SCAP (11); sebaceoma (9); TM (7); A/E D (3); VW (3); ESFA (2); IFK (1) | BCC (2) |
** the total number and/or lesion types were not fully described. AC – apocrine carcinoma, A/E D – apocrine/eccrine adenoma, AK – actinic keratosis, BCC – basal cell carcinoma, ESFA – eccrine syringofibroadenoma, IFK – inverted follicular keratosis, MAC – microcystic adnexal carcinoma, MN – melanocytic nevus, N – number, NA – not available, NS – nevus sebaceous, SCAP – syringocystadenoma papilliferum, SC – sebaceous carcinoma, SCC – squamous cell carcinoma, SK – seborrheic keratosis, TB – trichoblastoma, TE – trichoepithelioma, TFI – tumor of the follicular infundibulum, TM – trichilemmoma, VW – viral wart.
Seventeen studies reported the gender and anatomical distribution of NS. Males represented 52% of cases, and 91% of all cases were located on the head. Secondary lesions within NS were reported in 12% of cases, of which 10% (709) were benign, and nearly 2% (132) were malignant. Six different types of malignant neoplasms and over 20 types of benign lesions were identified. The most common benign lesion related to NS was syringocystadenoma papilliferum (SCAP) in 25% of cases, followed by trichoblastoma (19%), warts (2%), and trichilemmoma (2%). Among malignant tumors, BCC was the most frequently reported (79%), while SCC represented 3% of cases.
Regarding sex distribution, 52% of benign and 74% of malignant tumors were found in males. Age distribution data for benign tumors were available in 11 articles, showing that of 360 tumors, 81% occurred in adults, 9% in adolescents, and 3% in children. For malignant tumors, data from 13 articles represented 99 cases, with only 5 reported in adolescents and 2 in children. Multiple tumors within a single NS were presented in only 98 (1%) out of 4,522 patients reviewed in 19 articles.
Among the included studies, 26 were classified as having a low risk of bias, while only one study was considered low quality (table 2).
Table 2
Quality assessment of included studies using the Newcastle-Ottawa Scale
DISCUSSION
To our knowledge, this systematic review analyzes the largest cohort of patients with NS for secondary tumor rates, which was 12%. Consistent with recent publications, our results showed that most of these tumors were benign, predominantly SCAP, while only 2% were malignant lesions, primarily BCC [3–5]. Although the occurrence of multiple tumors in a single NS was rare, the simultaneous presence of benign and malignant subtypes was detected, which should be considered during incisional biopsies to avoid missing malignant diagnoses [6].
The formation of secondary tumors in nevus sebaceous (NS) is largely due to postzygotic activating mutations in RAS pathway, most often HRAS (c.37G, C), less frequently, KRAS or NRAS. These mutations cause constitutive activation of MAPK and PI3K pathways, which causes abnormal cellular proliferation. NS is thus categorized to be a mosaic RASopathy which is due to somatic mutations limited to lesional skin. In spite of the fact that these mutations occur at birth, tumor growth occurs later in life suggesting a multi-step model where other genetic factors i.e. mutation in TP53 or NOTCH2 may play a role in neoplastic transformation. However, the full spectrum and functional impact of these secondary mutations remain incompletely understood and require further investigation [7, 8].
We observed that NS-related malignancies were more frequent in male adults. However, the lack of control groups and adjustment for confounders in the included studies were limitations of our review. Factors such as UV exposure, genetic predispositions, and environmental influences were not properly differentiated in the cohorts, which may affect their incidences of malignancy [4].
CONCLUSIONS
Despite our efforts to identify specific characteristics that justify the prophylactic excision of SN in many cases, the overall low risk of malignancy raises questions about the necessity of such procedures for all patients, especially children [9]. Therefore, clinical management should consider each NS individually and its likelihood of tumor progression, evaluating the feasibility of regular monitoring versus additional surgical measures.