INTRODUCTION
Keratoacanthoma (KA) is a tumor that arises from hair follicle epithelium. It is characterized by rapid growth, tendency to local invasion and spontaneous regression [1]. Typically, the tumor reaches its final size of 10 to 25 mm within 6 months [2]. Classical and most common KA variant manifests as a solitary, sharply demarcated, firm, pinkish or skin-colored nodule or tumor with a central hyperkeratotic horn plug, the removal of which creates a crater-like structure [3, 4]. Less common variants are keratoacanthoma centrifugum marginatum, giant keratoacanthoma (GKA), eruptive keratoacanthoma and multiple keratoacanthomas associated with genetic syndromes [5, 6]. GKA is a rare variant of solitary keratoacanthoma and is characterized by a size greater than 20 mm. It has been diagnosed more often in men with a peak incidence in the fifth decade of life [7]. In the differential diagnosis, mainly cutaneous squamous cell carcinoma (SCC) should be considered [4].
OBJECTIVE
This article presents the case of a patient with a giant keratoacanthoma in the context of chronic myeloid leukemia.
CASE REPORTS
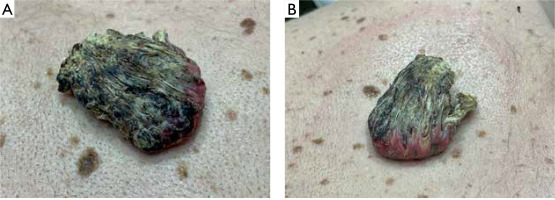
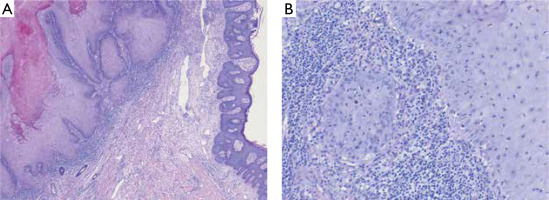
A 57-year-old man presented with a tender to palpation, large (10 × 6 cm in size) exophytic tumor with central hyperkeratotic masses located on his back, in the vertebral line of the interscapular region (figs. 1, A, B). The lesion had appeared approximately 2 years earlier and was growing with time. There was no history of preceding trauma. The patient suffered from chronic myeloid leukemia and schizophrenia, diagnosed over 25 years before. At the time of presentation, the patient was taking quetiapine and amisulpride. Besides, the clinical examination was insignificant. After obtaining the patient’s written consent, the lesion was surgically excised with 0.5 cm margin. Based on histopathological evaluation keratoacanthoma was diagnosed (fig. 2).
DISCUSSION
Giant KA is a rarely described tumor. According to the only report summarizing clinical and pathological features conducted by Bogner et al., GKA occurs predominantly on the face and limbs [8]. The largest GKA reported in a 55-year-old individual was located on the hand, developed within post-burn scar and measured 10 × 7 cm [1]. The characteristic feature of KA is the evolution of the tumor divided into three stages: rapid growth (4–14 weeks), stationary phase (2–8 weeks) and involution, usually lasting 4 months [9–13]. In the case of the described patient, the lesion gradually enlarged for 2 years and at the time of diagnosis did not show any signs of regression. Although a few similar cases of GKA with a longer history have been described, the exact evolution of giant tumors enabling clinical differentiation from SCC has not been known so far [8]. Similarly, the etiology and pathogenesis of the disease are debatable. The most common risk factors of KA include chronic exposure to ultraviolet (UV) and Roentgen radiation, contact with tar products, trauma, skin burns, human papillomavirus infection, certain medications (including PD-1/PD-L1, BRAF and Hedgehog pathway inhibitors, sorafenib, infliximab), as well as immunological disorders and post-infectious immunosuppression [14–20]. Among the described cases of giant tumors, almost all were located on the skin chronically exposed to UV radiation and several of them were associated with pre-existing diseases such as burn or hyperplastic lichen planus [8]. History of chronic myeloid leukemia was the most important risk factor for GKA in our patient. The histopathological features of KA are marked acanthosis, hyperkeratosis and a central, rimmed (or supported) keratin plug at the edges giving a characteristic crater-like appearance [8, 21]. KA, including giant lesions, may microscopically resemble well-differentiated SCC, but the lack of vascular and neural invasion and preservation of the basement membrane integrity help to distinguish these two entities [21]. The most common treatment method reported in the literature is surgery and combined surgical treatment with radiotherapy [8, 22, 23]. Intralesional injections of cytostatic drugs have also been studied in several reports to accelerate regression of the lesion [23]. However, the efficacy of pharmacological treatment is difficult to assess due to the possibility of spontaneous tumor regression. Another limitation is that available data is based on case series with the absence of randomized controlled trials [22, 23]. Considering diagnostic difficulties in differentiation with SCC, high risk of post-involution scarring and lack of sufficient knowledge about the biological behavior of GKA, surgical excision seems to be the best treatment option.