INTRODUCTION
Keratoacanthoma (KA) is a low-grade cutaneous neoplasm deriving from the hair follicle [1]. It is a 1–2 cm dome-shaped tumor known for rapid growth with characteristic central keratinous plug [1, 2]. KA usually occurs on sun-exposed areas such as the face and extremities in elderly individuals. It affects lighter skin colors (Fitzpatrick types I, II) more than others and has a close clinical and histopathological resemblance to squamous cell carcinoma (SCC) [1–3]. While KA often undergoes spontaneous involution, predicting its progression or involution is difficult [2]. KA can cause local tissue destruction if allowed to grow, so early treatment is recommended [1, 2]. Surgical excision remains the treatment of choice for most cases of KA [1, 2]. However, various treatment modalities exist, including radiation therapy, systemic oral retinoids, and intralesional applications of 5-fluorouracil (5-FU), methotrexate (MTX), or interferon α-2a [2, 3]. Surgical excision of KA can result in functional and cosmetic defects, particularly in cases of large lesions or those located in anatomically strategic areas [3]. In such cases, effective nonsurgical treatments are desirable. Several case reports have described the successful treatment of KA with intralesional MTX [3].
MTX is a chemical drug that inhibits DNA synthesis in actively dividing cells, making it appropriate for rapidly growing tumors [1]. It is a folic acid analog that binds to dihydrofolate reductase, blocking the formation of tetrahydrofolate and preventing the synthesis of the purine nucleotide thymidine [1–3]. Intralesional MTX is a potential alternative to surgical treatment of KAs, offering a less invasive and less costly treatment modality with decreased morbidity [2, 3].
OBJECTIVE
Main objective: To study the efficacy of intralesional MTX injections for the treatment of KA. Secondary objectives: To evaluate the efficacy of intralesional MTX injections for KA based on patient age and sex, as well as lesion duration and size.
MATERIAL AND METHODS
Patients
We treated 37 patients with KA in the Department of Dermatology at Tishreen University Hospital, Lattakia, Syria, between March 2022 and December 2023. The age of patients ranged from 36 to 93 years. The duration of lesions ranged from 1 to 28 weeks, and lesion sizes were between 10 to 30 mm. Variables recorded included patient age, sex, tumor size, lesion location, lesion duration, histologic confirmation of the diagnosis, cumulative MTX dose, total number of injections, interval between injections, treatment outcome, adverse events, and total patient follow-up time. Informed consent was obtained from all participants included in the study.
Methods
We used 15 mg of MTX for KA smaller than 20 mm, and 20 mg of MTX for KA larger than 20 mm. The product was provided by the manufacturer. MTX was injected at the base of the KA tumor using a 27-gauge needle at each treatment session. Shortly after the MTX injection, uniform tumor blanching was achieved. The injection site, the interval between injections, and the concentration and amount of MTX were adjusted based on the clinical response.
Study design
After approval by the local research ethics comittee (3642), a prospective (before and after) study was conducted in the Department of Dermatology and Venereology at Tishreen University Hospital in Lattakia, Syria, between 2022 and 2023.
Inclusion criteria
Patients with KA who visited Tishreen University Hospital between March 2022 and December 2023.
Exclusion criteria
Contraindications for methotrexate, included bone marrow suppression, liver dysfunction, kidney dysfunction, pregnancy, pulmonary dysfunction, severe infections, and peptic ulcer.
Research sample and data collection
The final research group consisted of 37 patients who underwent treatment with intralesional methotrexate. Data were collected prospectively, and all participants were fully informed about the procedure. Written informed consent was obtained from all participants or their families. This study did not encounter serious ethical challenges. Upon admission, participants underwent clinical and laboratory evaluation.
Clinical evaluation
A detailed clinical history was taken, and the following information was documented: age, sex, KA location, and size.
Clinical and laboratory examination
Before treatment, laboratory analyses were performed, such as a complete blood count with differential, liver enzymes (ALT and AST), and kidney function tests (creatinine and urea).
Treatment
The skin was sterilized with 4% dermal povidone. We used 15 mg of MTX for KA smaller than 20 mm, and 20 mg of MTX for KA larger than 20 mm.
MTX was diluted in normal saline (0.9%) using a 1 ml syringe.
MTX was injected at the base of the KA with a 27-gauge needle, starting from the peripheral edges of the lesion to the center until blanching of the lesion was achieved. Injections were repeated to involve all lesion edges, with suction applied prior to injection to avoid inadvertent intravascular administration. Patients received 10 mg of folic acid the day after treatment. Injections were repeated once weekly for a maximum of 8 weeks until lesions resolved completely.
Results
The research sample included 37 patients with KA who met the inclusion criteria. The patients’ ages ranged from 26 to 93 years, with a mean age of 65.52 ±13.2 years. Lesion duration ranged from 1 to 28 weeks, with an average duration of 8.58 ±5.4 weeks. The total dose of MTX ranged from 20 to 170 mg, with an average of 55.13 ±35.2 mg.
Among the patients, 78.4% were men and 21.6% were women (table 1). Lesions were located mostly on the face (51.4%) and the extremities (40.5%) (table 2). Tumor sizes were less than 20 mm in 83.8% of cases, with an average size of 14.81 ±5.1 mm (table 3).
Table 2
Keratoacanthoma location
| Keratoacanthoma area/location | Patient number | Ratio |
|---|---|---|
| Face | 19 | 51.4% |
| Extremities | 15 | 40.5% |
| Head | 2 | 5.4% |
| Trunk | 1 | 2.7% |
| Total | 37 | 100% |
Table 3
Keratoacanthoma size
| Keratoacanthoma size | Patient number | Ratio |
|---|---|---|
| < 20 mm | 31 | 83.8% |
| ≥ 20 mm | 6 | 16.2% |
| Total | 37 | 100% |
By the fourth session, the majority of lesions (93.45%) healed completely and by the end of the treatment sessions, the number of the healed lesions achieved 98.78%. However, 2 (5.4%) patients showed noticeable improvement without complete healing (tables 4 and 5).
Table 4
Treatment results
| Healing | Patient number | Ratio |
|---|---|---|
| Complete healing | 35 | 94.6% |
| Improvement | 2 | 5.4% |
| Total | 37 | 100% |
Table 5
Complete healing and improvement at the end of sessions
There were statistically significant differences in the mean values of KA size before and after treatment. Before treatment, the average size was 14.81 ±5.1 mm, which decreased to 0.18 ±0.8 mm after treatment, with a p-value of less than 0.001 (table 4).
No statistically significant differences were found regarding the average number of sessions until complete regression and sex, with men requiring an average of 3.03 ±1.9 sessions and women requiring 2.37 ±1.06 sessions (p = 0.3) (table 6). Additionally, no statistically significant differences were found between the number of sessions and patient age (p = 0.9) (table 7).
Table 6
Sessions needed according to patients’ sex
| Session parameter | Male | Female | P-value |
|---|---|---|---|
| Mean ± SD | 3.03 ±1.9 sessions | 2.37 ±1.06 sessions | 0.3 |
| Min.–max. (number of sessions) | 1–8 sessions | 1–4 sessions |
Table 7
Sessions needed according to patients’ age
However, there were statistically significant differences regarding the number of sessions and tumor size; larger tumors required more sessions to heal than smaller ones (table 8). Similarly, statistically significant differences were observed between the number of treatment sessions and tumor age; lesions of longer duration necessitated a greater number of sessions for complete healing compared to lesions with shorter duration (table 9). Finally, we found statistically significant differences in the number of sessions required for complete tumor regression based on tumor location.
Table 8
Sessions needed according to tumor size
| Keratoacanthoma size [mm] | Patient number | Number of sessions needed | P-value | Pearson correlation |
|---|---|---|---|---|
| < 10 | 10 | 1.32 ±0.85 | 0.0001 | 0.8 |
| 10–20 | 21 | 2.63 ±1.15 | ||
| > 20 | 6 | 3.27 ±2.4 |
Table 9
Sessions needed according to tumor duration
| Keratoacanthoma duration [weeks] | Keratoacanthoma number | Number of sessions needed | P-value | Pearson correlation |
|---|---|---|---|---|
| 0–4 | 10 | 1.65 ±1.1 | 0.001 | 0.33 |
| 4–8 | 17 | 2.60 ±1.53 | ||
| 8–12 | 7 | 3.21 ±2.05 | ||
| 12–24 | 3 | 4.17 ±2.73 |
Tumors on the face needed an average of 1.89 ±0.8 sessions to resolve, while tumors on the extremities required an average of 4.33 ±1.8 sessions (table 10, figs. 1–4).
Table 10
Sessions needed according to tumor location
| Session parameter | Face | Head | Extremities | Trunk | P-value |
|---|---|---|---|---|---|
| Mean ± SD | 1.89 ±0.8 | 2 ±1.4 | 4.33 ±1.8 | 2 ±0 | 0.0001 |
| Min.–max. (Session number) | 1–4 | 1–3 | 2–8 | 2–2 |
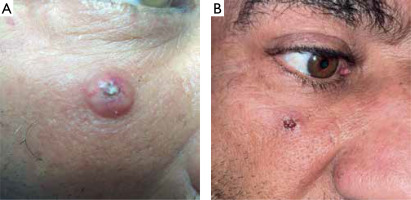
Figure 1
A – A 86-year-old woman with a 21 mm keratoacanthoma on the lower right eyelid. B – After 2 sessions (2 weeks) of treatment, with follow-up at 6 weeks
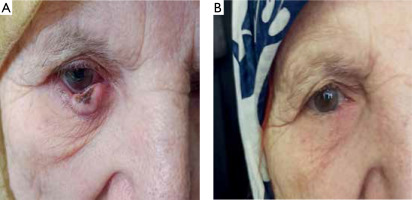
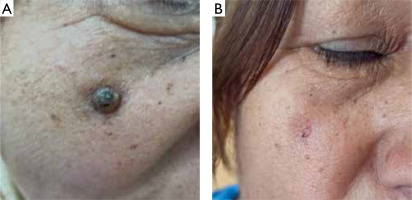
Figure 2
A – A 61-year-old man with a 12 mm keratoacanthoma on the nose. B – After 4 sessions (4 weeks) of treatment, with follow-up
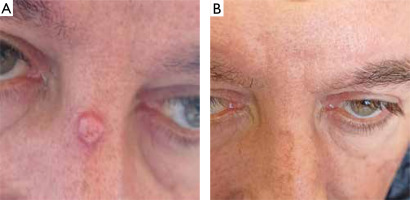
DISCUSSION
In this study, 37 patients with an average keratoacanthoma diameter of 1.8 cm were treated with an average of 3.4 sessions and a total MTX dose of 55.1 mg. The majority of tumors were located on the face (51%), followed by the extremities (40%), likely due to UV light exposure. Most of the tumors were less than 20 mm in size.
By the fourth session, 94.6% of tumors showed complete regression, and by the eighth session, 98.78% were completely regressed. However, 5.4% showed significant improvement without complete regression by the end of the treatment sessions. Tumors on the extremities took significantly longer to regress (4.3 sessions) compared to those on the face (1.8 sessions). Larger tumors required more sessions for regression than smaller ones, and short duration tumors took fewer sessions to regress compared to older ones. There was no significant correlation between tumor regression and the patient’s sex or age. No significant side effects were observed after the sessions, and no tumor recurrence was reported after 6 months of follow-up.
Since KA can regress spontaneously, some authorities argue that treatment is not always necessary [2]. However, KA may need a long time to involute spontaneously and may continue to enlarge during this period, destroying the involved tissues [4]. Multiple treatment alternatives exist for KA, with excisional surgery being the standard of care for large or high-risk cases [5]. Intralesional chemotherapy for non-melanoma skin cancer has existed for more than five decades [5]. Despite this, it is infrequently used, and recent consensus guidelines for the treatment of squamous cell carcinoma and basal cell carcinoma do not include intralesional chemotherapy [1]. Barriers to its use include the absence of therapeutic guidelines, off-label utilization of these agents, a relatively small number of treated patients, and a lack of large, well-designed trials with long-term follow-up [6].
Non-operative approaches may need to be considered in well-selected patients when poor cosmetic outcomes and loss of function are expected from surgery, or for those who cannot undergo surgery, such as elderly patients or those with comorbidities. In these cases, intralesional MTX offers a less invasive treatment option with acceptable cosmetic results [7]. This approach could also serve as neoadjuvant therapy to reduce tumor size before surgery, thereby reducing surgical invasiveness [7].
For our patients, we opted for intralesional MTX due to its effectiveness for solitary and multiple KAs and its relatively low risk of morbidity compared with surgical excision, particularly in sensitive regions such as the face, ears, and hands in elderly patients [8]. This simple procedure appears to be efficacious, painless, and safe, with no significant systemic adverse events [6]. Monitoring complete blood cell counts and liver enzyme levels at baseline and during treatment is recommended [9]. It would have been beneficial to continue monitoring for cytopenia and liver enzyme alterations during follow-up, especially in elderly patients or those with concomitant diseases [10].
Our study agreed with the study published by Scalvenzi et al. in 2019, which included 11 patients with KA treated weekly with 20-25 mg of intralesional methotrexate. All patients showed complete regression of tumors after 4–8 weeks [1].
Our study also aligned with the study published by Yoo and Kim in Korea in 2014, which included 11 patients treated with 12.5–25 mg of intralesional MTX once every 10 days for 2–7 sessions. By the end of the sessions, 91% of patients showed complete regression of tumors [2].
Furthermore, our study was consistent with the study published by Smith et al. in Texas, USA, in 2020. This study included 69 lesions treated with intralesional MTX every 2–4 weeks, with 95.7% of lesions showing complete regression [11].
CONCLUSIONS
Intralesional MTX is an effective treatment for keratoacanthoma. Tumors on the face respond to treatment faster than those in other locations.
RECOMMENDATIONS
Utilize intralesional MTX for the treatment of KA, particularly for tumors on the face and other sensitive locations where surgery may be difficult or result in malformed scars.
Conduct future studies with a larger sample of patients and include longer follow-up periods after treatment to further validate these findings.