Introduction
Numerous studies have been published to assess the quality of life (QoL) of patients after Cushing’s disease (CD) treatment. However, they mainly concern the adult population [1, 2]. Available data on the QoL have shown that patients with CD still experience significant problems in many areas of life, even after the achievement of biochemical remission and many years after the definitive treatment. Hypercortisolemia caused by CD, even when cured, may have long-term adverse effects on the mood and functioning in the society [1]. It was also proved that patients with CD have impaired mental well-being and psychosocial functioning compared to patients with other pituitary tumors [3–7]. Moreover, patients after successful CD treatment may still have a persistent increased cardiovascular risk, body composition alterations and depression, anxiety or impaired cognition [8, 9]. As pituitary deficits requiring hormonal substitution may be diagnosed after CD treatment, patients may complain of incomplete recovery, both physical and psychological, which affects their QoL [10]. The only prospective study conducted on the pediatric population by Keil et al. allows similar conclusions to be drawn [11].
Purpose
The aim of the presented study was to expand the knowledge about the QoL of adult patients treated due to CD in childhood.
Material and methods
Eighteen adult patients out of 29 patients diagnosed with CD and/or treated at our Institute between 1994 and 2018 took part in the survey. All patients gave a formal consent in accordance with the requirements of the Institutional Bioethical Committee (48/KBE/2018). All patients received the World Health Organization Questionnaire of the Quality of Life (WHOQOL-BREF). WHOQOL-BREF questionnaire details are mentioned in Supplemental Material 1, which contains also information about transformation of obtained results according to the appropriate key [12]. Selected sociodemographic data came from the author’s questionnaire completed by each patient. The influence of the following prognostic factors for the QoL has been analyzed:
selected data from the author’s questionnaire: relationship status, education, source of income, place of residence;
clinical factors: gender, age at disease onset, time from the onset of symptoms to pituitary surgery, hormonal status.
Hypopituitarism was defined as one or more pituitary hormone deficiencies requiring replacement therapy. Patients data were compared with a control group of 38 healthy subjects with the same age and sex distribution and from the same geographical area as analyzed patients.
Statistical analysis
Data were analyzed using Statistica 13.0 PL for Windows. The mean, range and standard deviation (SD) were calculated for continuous variables. The distribution of continuous variables was checked for normality. The Student t-test was used to determine significant differences for variables with a normal distribution and to compare patient and control data. Correlation analysis was used to determine the Pearson’s linear correlation coefficient r. The p-value of less than 0.05 was considered to be statistically significant.
Results
The study group included ten (55.56%) women and eight (44.44%) men, aged from 19.75 years to 40.33 years, with the mean age of 28.93 years. The sociodemographic characteristics of surveyed patients is shown in Table I. Regarding the marital status, fifty percent of the surveyed patients were married and the other half of the patients were single. Over half of the patients (55.56%) had a higher education degree (equivalent to obtaining a master’s degree). Regarding the source of income, over half of the patients (66.67%) reported to be professionally active, 11.11% of the patients were on a pension and 22.22% reported to have other sources of income. 66.67% of patients reported that they lived in an urban area.
Table I
The sociodemographic characteristics of the surveyed patients
As regards the analyzed clinical factors: the mean age at the disease onset was 10.93 years (6.5–16). The mean interval between the onset of symptoms and pituitary surgery was 3.82 years (0.82–9.08). The mean time from transsphenoidal surgery (TSS) to the date of completing the questionnaire was 14.13 years (5.42–24.50). Two (11.11%) patients had a single pituitary hormone deficiency and 12 (66.67%) patients had multiple pituitary hormone deficiencies. Four patients did not take any substitution therapy for pituitary deficiency.
The mean age of the control group was 30.84 ±2.67 years (10 males and 26 females). The age and sex were not different between patients and controls (p = 0.10 and p = 0.38, respectively). Table II shows the mean transformed scores in the range of 0–100 with the min., max. and median value for each item of WHOQOL-BREF in comparison with the control group and with Heald et al. study [1].
Table II
Mean Quality of Life scores in different domains (presented as transformed item scores in the range of 0-100) compared to the results of the control group and to the results presented in the study by Heald et al. [1] (mean ±SD*)
No significant difference in the QoL was noted between analyzed patients and controls (Table II). The mean scores of all domains and the total score of WHOQOL-BREF according to the sociodemographic and clinical parameters are presented in Table III.
Table III
The association of sociodemographic and clinical factors with WHOQOL-BREF domains (mean ±SD*)
No gender differentiation was observed in relation to the QoL in this study. No significant difference in the QoL was noted between patients in a relationship and patients who were single. Regarding education, no significant difference occurred between patients with different levels of education. However, patients with primary and/or secondary education had lower results in domain 2 (psychological health) in comparison with patients with an incomplete higher and/or higher education (p = 0.07). Professionally active people presented a higher QoL in domain 1 – physical health (p = 0.041) and domain 4 – environment (p = 0.016) than patients on a pension and those who reported other sources of income. Patients with hypopituitarism had lower results in domain 4 in comparison with patients without hypopituitarism (p = 0.31; Table III) and lower results in domain 2 in comparison with the control group (p = 0.045).
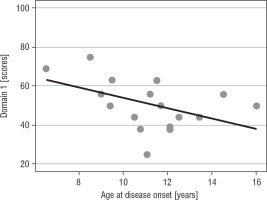
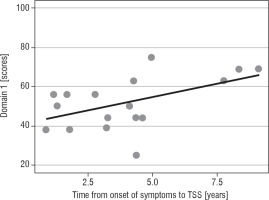
The higher age at disease onset, the lower QoL in domain 1 (p = 0.031) was reported (Fig. 1A). Patients with a longer interval between disease onset and TSS reported significantly more improvement in the physical domain (p = 0.029; Fig. 1B) and the environmental domain (p = 0.031; Table IV).
Table IV
The association of selected clinical factors with WHOQOL-BREF domains. Pearson’s linear correlation coefficient – correlation analysis; 95% CI
Discussion
Compared with our own controls, QoL of adult patients treated for CD in childhood was not significantly different in any domain. These data are different with the data presented by van Aken et al. [2] who presented physical and psychosocial impairments in adult patients treated for CD in adulthood. Our patients with hypopituitarism had reduced QoL in domain 4 in comparison with patients with normal pituitary function after CD treatment, while hypopituitarism did affect QoL in domain 2 (p = 0.045) compared to controls. These results are also contrasting with the results presented by van Aken et al. who showed that patients with hypopituitarism had impaired QoL for all items, whereas patients without hypopituitarism only scored worse for general fatigue [2].
Our data show that patients whose TSS was performed on average 14.13 years before are characterized by better physical and psychological health in comparison with a study by Heald et al. [1] conducted on 114 adult patients with benign pituitary tumors in relation to primary diagnosis. The study demonstrated much lower results in those domains (domain 1: 35 ±5 points vs. 51.56 ±13.09 in our study, and domain 2: 42 ±6 points vs. 55.94 ±13.43, respectively) in adult patients with the mean of 7.7 years after CD diagnosis. Furthermore, the patients in our study obtained better results in the environmental domain (66.83 ±12.36 points vs. 53 ±5) in comparison with patients in this study [1]. The cited study from 2004 demonstrated that patients with treated Cushing’s disease had significantly impaired psychological well-being and psychosocial functioning across all tested domains in comparison with patients with all other pituitary tumors [1]. Our study presents similar results – our patients had significantly lower results in comparison with patients with macroprolactinoma, acromegaly and patients with surgically-treated nonfunctioning adenoma in domains 1 and 2, and in domain 4 (environment) regarding patients with macroprolactinoma [1].
Moreover, Van Aken et al. in his analysis of 58 adults after the average of 13.4 years of follow-up emphasized that the general QoL was decreased in the patients, predominantly manifesting as weakened psychosocial and physical spheres, especially in those with hypopituitarism [2]. Similar conclusions are provided by the only prospective study by Keil et al. conducted on the pediatric population [11]. The study on 40 children with Cushing’s syndrome (CS) including 34 children with CD demonstrated that pediatric CS was associated with impaired health-related QoL one year after successful treatment [11]. Post-TSS scores of all CS patients in this study were compared to normative data in the United States. The results remained significantly lower for: physical function (p < 0.02), role-physical (p < 0.02), global health perception (p < 0.001), emotional impact (parent) (p < 0.001) and physical summary score (p < 0.001) [11]. The presented results allow to draw similar conclusions as those included in the study by Van der Klaauw et al., who reported that patients with CD experienced impairment in the physical functioning in comparison with patients who had been surgically treated for nonfunctioning adenomas [13]. Furthermore, Van der Klaauw et al. reported that adult patients with CD observed significantly higher impairment in all areas of health-related QoL in comparison with other pituitary adenomas (acromegaly, prolactinoma, nonfunctioning adenomas) [13].
Different determinants have been described that impact the quality of life [2, 14–17]. The study by Van Aken et al. conducted on 58 adults revealed that age, age at diagnosis, gender and hypopituitarism are the independent determinants of the QoL after successful CD treatment [2]. Age at diagnosis negatively influenced physical functioning and physical role limitations (short form – 36 questionnaire (SF-36)), with a positive effect on changes in health (SF-36). Male patients obtained higher scores for motivation and activation compared to female patients (Multidimensional Fatigue Index) [2]. In studies by Valassi et al. [17] and Papoian et al. [18] a long diagnostic delay had a negative influence on the QoL with a higher age at diagnosis as an additional factor in the study by Valassi et al.
Our study demonstrated that a higher age at the disease onset negatively correlated with the QoL but only in the physical domain (p = 0.031). This finding is consistent with studies by Van Aken et al. and Valassi et al. [2, 17]. In contrast to the study by Valassi et al. and Papoian et al., a longer interval between disease onset and TSS (which may be comparable with the diagnostic delay in those studies) significantly improved QoL in the physical (p = 0.029) and the environmental domain (p = 0.031) in the present study [17, 18]. Moreover, working patients demonstrated a significantly higher QoL in domain 1 (p = 0.041) and domain 4 (p = 0.016) in comparison with (altogether) patients on a pension and those with other sources of income.
Our study did not reveal any significant differences between males and females in all four domains of the QoL, which stays in line with other articles [18–20]. However, some studies indicated the female gender as a determinant of the low QoL [2, 14–16, 21]. In the study conducted on 58 patients with cured Cushing’s disease Van Aken et al. reported that women scored lower compared to male patients in several fatigue scales: reduced activity (p < 0.001), reduced motivation (p < 0.001), and mental fatigue (p < 0.004). Similarly, in the Nottingham Health Profile, energy was reduced in female patients (p < 0.006) [2]. A study by Wagenmakers et al. carried out in 123 patients in remission of CS (98 patients in remission of CD) demonstrated lower results of the QoL in women in all dimensions of the QoL [21]. It was hypothesized that gender affects CD presentation and the long-term effects of hypercortisolism, but the published data are not consistent [21, 22] and further research is necessary.
The limitation of our study is the fact that the questionnaire that was applied is used to study the general aspects of health-related quality of life and is not specific for CD or CS patients as for example a disease-generated questionnaire CushingQoL [23]. However, the usage of WHOQOL-BREF was helpful to make comparisons between patients with different kinds of pituitary adenomas and, thanks to the wide application of this questionnaire, the obtained results can be compared with other diseases. A big advantage of the presented study is the fact that it is the first study that demonstrates the results of the QoL assessment in adult patients after CD treatment in childhood.
Conclusions
During long-term follow-up the QoL of patients after CD treatment in childhood is not significantly different wit QoL of healthy controls with the same age and sex distribution. The presence of hypopituitarism may negatively influence some aspects of QoL in these patients. Further studies are needed to expand the knowledge of factors that may contribute to the QoL in CD patients who were treated in childhood.

 ENGLISH
ENGLISH